Local Application of Leptin Antagonist Attenuates Angiotensin II-Induced Ascending Aortic Aneurysm and Cardiac Remodeling
- PMID: 27143353
- PMCID: PMC4889208
- DOI: 10.1161/JAHA.116.003474
Local Application of Leptin Antagonist Attenuates Angiotensin II-Induced Ascending Aortic Aneurysm and Cardiac Remodeling
Abstract
Background: Ascending thoracic aortic aneurysm (ATAA) is driven by angiotensin II (AngII) and contributes to the development of left ventricular (LV) remodeling through aortoventricular coupling. We previously showed that locally available leptin augments AngII-induced abdominal aortic aneurysms in apolipoprotein E-deficient mice. We hypothesized that locally synthesized leptin mediates AngII-induced ATAA.
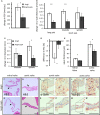
Methods and results: Following demonstration of leptin synthesis in samples of human ATAA associated with different etiologies, we modeled in situ leptin expression in apolipoprotein E-deficient mice by applying exogenous leptin on the surface of the ascending aorta. This treatment resulted in local aortic stiffening and dilation, LV hypertrophy, and thickening of aortic/mitral valve leaflets. Similar results were obtained in an AngII-infusion ATAA mouse model. To test the dependence of AngII-induced aortic and LV remodeling on leptin activity, a leptin antagonist was applied to the ascending aorta in AngII-infused mice. Locally applied single low-dose leptin antagonist moderated AngII-induced ascending aortic dilation and protected mice from ATAA rupture. Furthermore, LV hypertrophy was attenuated and thickening of aortic valve leaflets was moderated. Last, analysis of human aortic valve stenosis leaflets revealed de novo leptin synthesis, whereas exogenous leptin stimulated proliferation and promoted mineralization of human valve interstitial cells in culture.
Conclusions: AngII-induced ATAA is mediated by locally synthesized leptin. Aortoventricular hemodynamic coupling drives LV hypertrophy and promotes early aortic valve lesions, possibly mediated by valvular in situ leptin synthesis. Clinical implementation of local leptin antagonist therapy may attenuate AngII-induced ATAA and moderate related LV hypertrophy and pre-aortic valve stenosis lesions.
Clinical trial registration: URL: https://www.clinicaltrials.gov/. Unique identifier: NCT00449306.
Keywords: angiotensin II; aortic aneurysm; aortic valve stenosis; left ventricular hypertrophy; leptin; leptin antagonist; vascular remodeling.
© 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures






Similar articles
-
I-κB kinase-ε knockout protects against angiotensin II induced aortic valve thickening in apolipoprotein E deficient mice.Biomed Pharmacother. 2019 Jan;109:1287-1295. doi: 10.1016/j.biopha.2018.10.083. Epub 2018 Nov 9. Biomed Pharmacother. 2019. PMID: 30463808
-
Localized Antileptin Therapy Prevents Aortic Root Dilatation and Preserves Left Ventricular Systolic Function in a Murine Model of Marfan Syndrome.J Am Heart Assoc. 2020 May 18;9(10):e014761. doi: 10.1161/JAHA.119.014761. Epub 2020 May 7. J Am Heart Assoc. 2020. PMID: 32378446 Free PMC article.
-
Rapid dilation of the abdominal aorta during infusion of angiotensin II detected by noninvasive high-frequency ultrasonography.J Vasc Surg. 2006 Aug;44(2):372-6. doi: 10.1016/j.jvs.2006.04.047. J Vasc Surg. 2006. PMID: 16890871
-
Calcific Aortic Valve Disease: Part 2-Morphomechanical Abnormalities, Gene Reexpression, and Gender Effects on Ventricular Hypertrophy and Its Reversibility.J Cardiovasc Transl Res. 2016 Aug;9(4):374-99. doi: 10.1007/s12265-016-9695-z. Epub 2016 May 16. J Cardiovasc Transl Res. 2016. PMID: 27184804 Free PMC article. Review.
-
The Effect of Ascending Aortic Repair on Left Ventricular Remodeling.Am J Cardiol. 2022 Nov 1;182:89-94. doi: 10.1016/j.amjcard.2022.07.027. Epub 2022 Sep 6. Am J Cardiol. 2022. PMID: 36068098 Review.
Cited by
-
Telmisartan improves myocardial remodeling by inhibiting leptin autocrine activity and activating PPARγ.Exp Biol Med (Maywood). 2020 Apr;245(7):654-666. doi: 10.1177/1535370220908215. Epub 2020 Feb 19. Exp Biol Med (Maywood). 2020. PMID: 32075431 Free PMC article.
-
Association Between Serum Leptin Level and Calcific Aortic Valve Disease.J Am Heart Assoc. 2019 Oct;8(19):e012495. doi: 10.1161/JAHA.119.012495. Epub 2019 Sep 28. J Am Heart Assoc. 2019. PMID: 31566104 Free PMC article.
-
Human Perivascular Adipose Tissue as a Regulator of the Vascular Microenvironment and Diseases of the Coronary Artery and Aorta.J Cardiol Cardiovasc Sci. 2019;3(4):10-15. doi: 10.29245/2578-3025/2019/4.1174. Epub 2019 Aug 13. J Cardiol Cardiovasc Sci. 2019. PMID: 32411947 Free PMC article.
-
Effect of QSKL on MAPK and RhoA Pathways in a Rat Model of Heart Failure.Evid Based Complement Alternat Med. 2017;2017:3903898. doi: 10.1155/2017/3903898. Epub 2017 Apr 18. Evid Based Complement Alternat Med. 2017. PMID: 28484504 Free PMC article.
-
Postoperative Dynamic of Leptin and Fibroblast Growth Factor 21 in 123 Patients Recovering from Cardiac Surgery.Med Sci Monit. 2022 Sep 29;28:e937652. doi: 10.12659/MSM.937652. Med Sci Monit. 2022. PMID: 36171690 Free PMC article.
References
-
- Davies RR, Goldstein LJ, Coady MA, Tittle SL, Rizzo JA, Kopf GS, Elefteriades JA. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17–27; discussion 27‐8. - PubMed
-
- Danyi P, Elefteriades JA, Jovin IS. Medical therapy of thoracic aortic aneurysms: are we there yet? Circulation. 2011;124:1469–1476. - PubMed
-
- Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111:816–828. - PubMed
-
- Tadros TM, Klein MD, Shapira OM. Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications. Circulation. 2009;119:880–890. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

