Suppression of Antitumor Immune Responses by Human Papillomavirus through Epigenetic Downregulation of CXCL14
- PMID: 27143385
- PMCID: PMC4959654
- DOI: 10.1128/mBio.00270-16
Suppression of Antitumor Immune Responses by Human Papillomavirus through Epigenetic Downregulation of CXCL14
Abstract
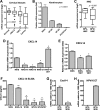
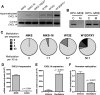
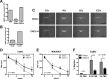
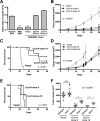
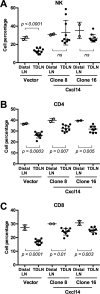
High-risk human papillomaviruses (HPVs) are causally associated with multiple human cancers. Previous studies have shown that the HPV oncoprotein E7 induces immune suppression; however, the underlying mechanisms remain unknown. To understand the mechanisms by which HPV deregulates host immune responses in the tumor microenvironment, we analyzed gene expression changes of all known chemokines and their receptors using our global gene expression data sets from human HPV-positive and -negative head/neck cancer and cervical tissue specimens in different disease stages. We report that, while many proinflammatory chemokines increase expression throughout cancer progression, CXCL14 is dramatically downregulated in HPV-positive cancers. HPV suppression of CXCL14 is dependent on E7 and associated with DNA hypermethylation in the CXCL14 promoter. Using in vivo mouse models, we revealed that restoration of Cxcl14 expression in HPV-positive mouse oropharyngeal carcinoma cells clears tumors in immunocompetent syngeneic mice, but not in Rag1-deficient mice. Further, Cxcl14 reexpression significantly increases natural killer (NK), CD4(+) T, and CD8(+) T cell infiltration into the tumor-draining lymph nodes in vivo In vitro transwell migration assays show that Cxcl14 reexpression induces chemotaxis of NK, CD4(+) T, and CD8(+) T cells. These results suggest that CXCL14 downregulation by HPV plays an important role in suppression of antitumor immune responses. Our findings provide a new mechanistic understanding of virus-induced immune evasion that contributes to cancer progression.
Importance: Human papillomaviruses (HPVs) are causally associated with more than 5% of all human cancers. During decades of cancer progression, HPV persists, evading host surveillance. However, little is known about the immune evasion mechanisms driven by HPV. Here we report that the chemokine CXCL14 is significantly downregulated in HPV-positive head/neck and cervical cancers. Using patient tissue specimens and cultured keratinocytes, we found that CXCL14 downregulation is linked to CXCL14 promoter hypermethylation induced by the HPV oncoprotein E7. Restoration of Cxcl14 expression in HPV-positive cancer cells clears tumors in immunocompetent syngeneic mice, but not in immunodeficient mice. Mice with Cxcl14 reexpression show dramatically increased natural killer and T cells in the tumor-draining lymph nodes. These results suggest that epigenetic downregulation of CXCL14 by HPV plays an important role in suppressing antitumor immune responses. Our findings may offer novel insights to develop preventive and therapeutic tools for restoring antitumor immune responses in HPV-infected individuals.
Copyright © 2016 Cicchini et al.
Figures

References
-
- Bergot AS, Ford N, Leggatt GR, Wells JW, Frazer IH, Grimbaldeston MA. 2014. HPV16-E7 expression in squamous epithelium creates a local immune suppressive environment via CCL2- and CCL5-mediated recruitment of mast cells. PLoS Pathog 10:e00270-16. doi: 10.1371/journal.ppat.1004466. - DOI - PMC - PubMed
-
- den Boon JA, Pyeon D, Wang SS, Horswill M, Schiffman M, Sherman M, Zuna RE, Wang Z, Hewitt SM, Pearson R, Schott M, Chung L, He Q, Lambert P, Walker J, Newton MA, Wentzensen N, Ahlquist P. 2015. Molecular transitions from papillomavirus infection to cervical precancer and cancer: role of stromal estrogen receptor signaling. Proc Natl Acad Sci U S A 112:E3255–E3264. doi: 10.1073/pnas.1509322112. - DOI - PMC - PubMed
-
- Pyeon D, Newton MA, Lambert PF, den Boon JA, Sengupta S, Marsit CJ, Woodworth CD, Connor JP, Haugen TH, Smith EM, Kelsey KT, Turek LP, Ahlquist P. 2007. Fundamental differences in cell cycle deregulation in human papillomavirus-positive and human papillomavirus-negative head/neck and cervical cancers. Cancer Res 67:4605–4619. doi: 10.1158/0008-5472.CAN-06-3619. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

