Diurnal Regulation of Cellular Processes in the Cyanobacterium Synechocystis sp. Strain PCC 6803: Insights from Transcriptomic, Fluxomic, and Physiological Analyses
- PMID: 27143387
- PMCID: PMC4959675
- DOI: 10.1128/mBio.00464-16
Diurnal Regulation of Cellular Processes in the Cyanobacterium Synechocystis sp. Strain PCC 6803: Insights from Transcriptomic, Fluxomic, and Physiological Analyses
Abstract
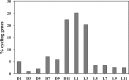
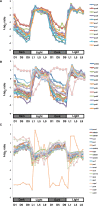
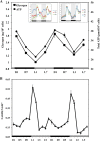
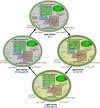
Synechocystis sp. strain PCC 6803 is the most widely studied model cyanobacterium, with a well-developed omics level knowledgebase. Like the lifestyles of other cyanobacteria, that of Synechocystis PCC 6803 is tuned to diurnal changes in light intensity. In this study, we analyzed the expression patterns of all of the genes of this cyanobacterium over two consecutive diurnal periods. Using stringent criteria, we determined that the transcript levels of nearly 40% of the genes in Synechocystis PCC 6803 show robust diurnal oscillating behavior, with a majority of the transcripts being upregulated during the early light period. Such transcripts corresponded to a wide array of cellular processes, such as light harvesting, photosynthetic light and dark reactions, and central carbon metabolism. In contrast, transcripts of membrane transporters for transition metals involved in the photosynthetic electron transport chain (e.g., iron, manganese, and copper) were significantly upregulated during the late dark period. Thus, the pattern of global gene expression led to the development of two distinct transcriptional networks of coregulated oscillatory genes. These networks help describe how Synechocystis PCC 6803 regulates its metabolism toward the end of the dark period in anticipation of efficient photosynthesis during the early light period. Furthermore, in silico flux prediction of important cellular processes and experimental measurements of cellular ATP, NADP(H), and glycogen levels showed how this diurnal behavior influences its metabolic characteristics. In particular, NADPH/NADP(+) showed a strong correlation with the majority of the genes whose expression peaks in the light. We conclude that this ratio is a key endogenous determinant of the diurnal behavior of this cyanobacterium.
Importance: Cyanobacteria are photosynthetic microbes that use energy from sunlight and CO2 as feedstock. Certain cyanobacterial strains are amenable to facile genetic manipulation, thus enabling synthetic biology and metabolic engineering applications. Such strains are being developed as a chassis for the sustainable production of food, feed, and fuel. To this end, a holistic knowledge of cyanobacterial physiology and its correlation with gene expression patterns under the diurnal cycle is warranted. In this report, a genomewide transcriptional analysis of Synechocystis PCC 6803, the most widely studied model cyanobacterium, sheds light on the global coordination of cellular processes during diurnal periods. Furthermore, we found that, in addition to light, the redox level of NADP(H) is an important endogenous regulator of diurnal entrainment of Synechocystis PCC 6803.
Copyright © 2016 Saha et al.
Figures

References
-
- Huang J, Wang J, Xu H. 2014. The circadian rhythms of photosynthesis, ATP content and cell division in Microcystis aeruginosa PCC7820. Acta Physiol Plant 36:3315–3323. doi:10.1007/s11738-014-1699-1. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

