Structural and transcriptomic response to antenatal corticosteroids in an Erk3-null mouse model of respiratory distress
- PMID: 27143398
- PMCID: PMC5003661
- DOI: 10.1016/j.ajog.2016.04.043
Structural and transcriptomic response to antenatal corticosteroids in an Erk3-null mouse model of respiratory distress
Abstract
Background: Neonatal respiratory distress syndrome in preterm infants is a leading cause of neonatal death. Pulmonary insufficiency-related infant mortality rates have improved with antenatal glucocorticoid treatment and neonatal surfactant replacement. However, the mechanism of glucocorticoid-promoted fetal lung maturation is not understood fully, despite decades of clinical use. We previously have shown that genetic deletion of Erk3 in mice results in growth restriction, cyanosis, and early neonatal lethality because of pulmonary immaturity and respiratory distress. Recently, we demonstrated that the addition of postnatal surfactant administration to antenatal dexamethasone treatment resulted in enhanced survival of neonatal Erk3-null mice.
Objective: To better understand the molecular underpinnings of corticosteroid-mediated lung maturation, we used high-throughput transcriptomic and high-resolution morphologic analysis of the murine fetal lung. We sought to examine the alterations in fetal lung structure and function that are associated with neonatal respiratory distress and antenatal glucocorticoid treatment.
Study design: Dexamethasone (0.4 mg/kg) or saline solution was administered to pregnant dams on embryonic days 16.5 and 17.5. Fetal lungs were collected and analyzed by microCT and RNA-seq for differential gene expression and pathway interactions with genotype and treatment. Results from transcriptomic analysis guided further investigation of candidate genes with the use of immunostaining in murine and human fetal lung tissue.
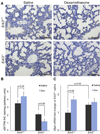
Results: Erk3(-/-) mice exhibited atelectasis with decreased overall porosity and saccular space relative to wild type, which was ameliorated by glucocorticoid treatment. Of 596 differentially expressed genes (q < 0.05) that were detected by RNA-seq, pathway analysis revealed 36 genes (q < 0.05) interacting with dexamethasone, several with roles in lung development, which included corticotropin-releasing hormone and surfactant protein B. Corticotropin-releasing hormone protein was detected in wild-type and Erk3(-/-) lungs at E14.5, with significantly temporally altered expression through embryonic day 18.5. Antenatal dexamethasone attenuated corticotropin-releasing hormone at embryonic day 18.5 in both wild-type and Erk3(-/-) lungs (0.56-fold and 0.67-fold; P < .001). Wild type mice responded to glucocorticoid administration with increased pulmonary surfactant protein B (P = .003). In contrast, dexamethasone treatment in Erk3(-/-) mice resulted in decreased surfactant protein B (P = .012). In human validation studies, we confirmed that corticotropin-releasing hormone protein is present in the fetal lung at 18 weeks of gestation and increases in expression with progression towards viability (22 weeks of gestation; P < .01).
Conclusion: Characterization of whole transcriptome gene expression revealed glucocorticoid-mediated regulation of corticotropin-releasing hormone and surfactant protein B via Erk3-independent and -dependent mechanisms, respectively. We demonstrated for the first time the expression and temporal regulation of corticotropin-releasing hormone protein in midtrimester human fetal lung. This unique model allows the effects of corticosteroids on fetal pulmonary morphologic condition to be distinguished from functional gene pathway regulation. These findings implicate Erk3 as a potentially important molecular mediator of antenatal glucocorticoid action in promoting surfactant protein production in the preterm neonatal lung and expanding our understanding of key mechanisms of clinical therapy to improve neonatal survival.
Keywords: CRH; ERK3; SFTPB; antenatal glucocorticoid; fetal lung maturation.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no conflict of interest.
Figures



Similar articles
-
Administration of antenatal glucocorticoids and postnatal surfactant ameliorates respiratory distress syndrome-associated neonatal lethality in Erk3(-/-) mouse pups.Pediatr Res. 2014 Jul;76(1):24-32. doi: 10.1038/pr.2014.54. Epub 2014 Apr 14. Pediatr Res. 2014. PMID: 24732107 Free PMC article.
-
Low-dose antenatal betamethasone treatment achieves preterm lung maturation equivalent to that of the World Health Organization dexamethasone regimen but with reduced endocrine disruption in a sheep model of pregnancy.Am J Obstet Gynecol. 2022 Dec;227(6):903.e1-903.e16. doi: 10.1016/j.ajog.2022.06.058. Epub 2022 Jul 2. Am J Obstet Gynecol. 2022. PMID: 35792176
-
Loss of Erk3 function in mice leads to intrauterine growth restriction, pulmonary immaturity, and neonatal lethality.Proc Natl Acad Sci U S A. 2009 Sep 29;106(39):16710-5. doi: 10.1073/pnas.0900919106. Epub 2009 Sep 15. Proc Natl Acad Sci U S A. 2009. PMID: 19805361 Free PMC article.
-
[Dilemmas about the antenatal use of corticosteroids for prevention of neonatal morbidity and mortality].Acta Med Croatica. 2005;59(2):129-35. Acta Med Croatica. 2005. PMID: 15909887 Review. Croatian.
-
Is there a role for antenatal TRH therapy for the prevention of neonatal lung disease?Semin Perinatol. 2001 Dec;25(6):406-16. doi: 10.1053/sper.2001.29032. Semin Perinatol. 2001. PMID: 11778911 Review.
Cited by
-
A Genome-Wide Association Study in Early COPD: Identification of One Major Susceptibility Loci.Int J Chron Obstruct Pulmon Dis. 2020 Nov 17;15:2967-2975. doi: 10.2147/COPD.S269263. eCollection 2020. Int J Chron Obstruct Pulmon Dis. 2020. PMID: 33235445 Free PMC article.
-
The effect of steroid administration on fetal diaphragm function.BMC Pregnancy Childbirth. 2022 Oct 12;22(1):762. doi: 10.1186/s12884-022-05074-3. BMC Pregnancy Childbirth. 2022. PMID: 36224559 Free PMC article.
-
Ciclesonide activates glucocorticoid signaling in neonatal rat lung but does not trigger adverse effects in the cortex and cerebellum.Neurobiol Dis. 2021 Aug;156:105422. doi: 10.1016/j.nbd.2021.105422. Epub 2021 Jun 11. Neurobiol Dis. 2021. PMID: 34126164 Free PMC article.
-
Maternal and early life exposures and their potential to influence development of the microbiome.Genome Med. 2022 Jan 11;14(1):4. doi: 10.1186/s13073-021-01005-7. Genome Med. 2022. PMID: 35016706 Free PMC article. Review.
-
The maternal plasma proteome changes as a function of gestational age in normal pregnancy: a longitudinal study.Am J Obstet Gynecol. 2017 Jul;217(1):67.e1-67.e21. doi: 10.1016/j.ajog.2017.02.037. Epub 2017 Mar 3. Am J Obstet Gynecol. 2017. PMID: 28263753 Free PMC article.
References
-
- Harris Requejo J. The State of the World’s Children 2009: Maternal and Newborn Health. [Accessed: March 4, 2016];2009 By Unicef Available at: http://books.google.com/books?id=EOZ6DKNBkSAC&lpg=PA75&ots=VKKRqaf1Sp&dq....
-
- Liggins GC. Premature delivery of foetal lambs infused with glucocorticoids. J Endocrinol. 1969;45(4):515–523. - PubMed
-
- The effect of antenatal steroids for fetal maturation on perinatal outcomes. [Accessed: March 4, 2016];NIH Consensus Statement. 1995 Feb-Mar;12(2):1–24. Available at: http://consensus.nih.gov/1994/1994AntenatalSteroidPerinatal095html.htm. - PubMed
-
- Pinkerton KE, Willet KE, Peake JL, Sly PD, Jobe AH, Ikegami M. Prenatal glucocorticoid and T4 effects on lung morphology in preterm lambs. Am J Respir Crit Care Med. 1997;156:624–630. - PubMed
-
- Warshamana GS, Martinez S, Lasky JA, Corti M, Brody AR. Dexamethasone activates expression of the PDGF-alpha receptor and induces lung fibroblast proliferation. Am J Physiol. 1998;274:L499–L507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

