Progesterone exerts neuroprotective effects and improves long-term neurologic outcome after intracerebral hemorrhage in middle-aged mice
- PMID: 27143417
- PMCID: PMC4857017
- DOI: 10.1016/j.neurobiolaging.2016.02.029
Progesterone exerts neuroprotective effects and improves long-term neurologic outcome after intracerebral hemorrhage in middle-aged mice
Erratum in
-
Corrigendum to "Progesterone exerts neuroprotective effects and improves long-term neurologic outcome after intracerebral hemorrhage in middle-aged mice" [Neurobiol. Aging 42 (2016) 13-24].Neurobiol Aging. 2021 Dec;108:236-237. doi: 10.1016/j.neurobiolaging.2021.09.005. Epub 2021 Oct 2. Neurobiol Aging. 2021. PMID: 34610867 No abstract available.
Abstract
In this study, we examined the effect of progesterone on histopathologic and functional outcomes of intracerebral hemorrhage (ICH) in 10- to 12-month-old mice. Progesterone or vehicle was administered by intraperitoneal injection 1 hour after collagenase-induced ICH and then by subcutaneous injections at 6, 24, and 48 hours. Oxidative and nitrosative stress were assayed at 12 hours post-ICH. Injury markers were examined on day 1, and lesion was examined on day 3. Neurologic deficits were examined for 28 days. Progesterone posttreatment reduced lesion volume, brain swelling, edema, and cell degeneration and improved long-term neurologic function. These protective effects were associated with reductions in protein carbonyl formation, protein nitrosylation, and matrix metalloproteinase-9 activity and attenuated cellular and molecular inflammatory responses. Progesterone also reduced vascular endothelial growth factor expression, increased neuronal-specific Na(+)/K(+) ATPase ɑ3 subunit expression, and reduced protein kinase C-dependent Na(+)/K(+) ATPase phosphorylation. Furthermore, progesterone reduced glial scar thickness, myelin loss, brain atrophy, and residual injury volume on day 28 after ICH. With multiple brain targets, progesterone warrants further investigation for its potential use in ICH therapy.
Keywords: Inflammatory response; Intracerebral hemorrhage; Neurologic function; Neuroprotective effects; Progesterone.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no disclosures relevant to the manuscript.
Figures

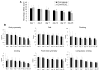

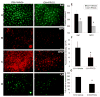



Similar articles
-
Taurine supplementation reduces neuroinflammation and protects against white matter injury after intracerebral hemorrhage in rats.Amino Acids. 2018 Apr;50(3-4):439-451. doi: 10.1007/s00726-017-2529-8. Epub 2017 Dec 18. Amino Acids. 2018. PMID: 29256178
-
Neuroprotective effects of hydrogen inhalation in an experimental rat intracerebral hemorrhage model.Brain Res Bull. 2018 Sep;142:122-128. doi: 10.1016/j.brainresbull.2018.07.006. Epub 2018 Jul 18. Brain Res Bull. 2018. PMID: 30016724
-
Dexmedetomidine Protects Against Neurological Dysfunction in a Mouse Intracerebral Hemorrhage Model by Inhibiting Mitochondrial Dysfunction-Derived Oxidative Stress.J Stroke Cerebrovasc Dis. 2019 May;28(5):1281-1289. doi: 10.1016/j.jstrokecerebrovasdis.2019.01.016. Epub 2019 Feb 20. J Stroke Cerebrovasc Dis. 2019. PMID: 30797643
-
Matrix metalloproteinase-9: dual role and temporal profile in intracerebral hemorrhage.J Stroke Cerebrovasc Dis. 2014 Nov-Dec;23(10):2498-2505. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.005. Epub 2014 Oct 11. J Stroke Cerebrovasc Dis. 2014. PMID: 25306400 Review.
-
White matter repair and treatment strategy after intracerebral hemorrhage.CNS Neurosci Ther. 2019 Oct;25(10):1113-1125. doi: 10.1111/cns.13226. Epub 2019 Oct 2. CNS Neurosci Ther. 2019. PMID: 31578825 Free PMC article. Review.
Cited by
-
Neurotrophic factor-based pharmacological approaches in neurological disorders.Neural Regen Res. 2023 Jun;18(6):1220-1228. doi: 10.4103/1673-5374.358619. Neural Regen Res. 2023. PMID: 36453397 Free PMC article. Review.
-
Inhibition of sugar-binding activity of Galectins-8 by thiogalactoside (TDG) attenuates secondary brain damage and improves long-term prognosis following intracerebral hemorrhage.Heliyon. 2024 Apr 26;10(9):e30422. doi: 10.1016/j.heliyon.2024.e30422. eCollection 2024 May 15. Heliyon. 2024. PMID: 38737270 Free PMC article.
-
Diagnostic and therapeutic use of oral micronized progesterone in endocrinology.Rev Endocr Metab Disord. 2024 Aug;25(4):751-772. doi: 10.1007/s11154-024-09882-0. Epub 2024 Apr 23. Rev Endocr Metab Disord. 2024. PMID: 38652231 Free PMC article. Review.
-
BYHWD Alleviates Inflammatory Response by NIK-Mediated Repression of the Noncanonical NF-κB Pathway During ICH Recovery.Front Pharmacol. 2021 May 7;12:632407. doi: 10.3389/fphar.2021.632407. eCollection 2021. Front Pharmacol. 2021. PMID: 34025405 Free PMC article.
-
Changes in motor function, cognition, and emotion-related behavior after right hemispheric intracerebral hemorrhage in various brain regions of mouse.Brain Behav Immun. 2018 Mar;69:568-581. doi: 10.1016/j.bbi.2018.02.004. Epub 2018 Feb 16. Brain Behav Immun. 2018. PMID: 29458197 Free PMC article.
References
-
- Barha CK, Ishrat T, Epp JR, Galea LA, Stein DG. Progesterone treatment normalizes the levels of cell proliferation and cell death in the dentate gyrus of the hippocampus after traumatic brain injury. Experimental neurology. 2011;231(1):72–81. doi: 10.1016/j.expneurol.2011.05.016. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

