Loss of CTRP5 improves insulin action and hepatic steatosis
- PMID: 27143553
- PMCID: PMC4935138
- DOI: 10.1152/ajpendo.00010.2016
Loss of CTRP5 improves insulin action and hepatic steatosis
Abstract
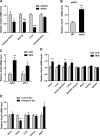
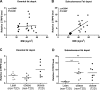
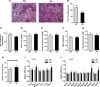
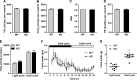
The gene that encodes C1q/TNF-related protein 5 (CTRP5), a secreted protein of the C1q family, is mutated in individuals with late-onset retinal degeneration. CTRP5 is widely expressed outside the eye and also circulates in plasma. Its physiological role in peripheral tissues, however, has yet to be elucidated. Here, we show that Ctrp5 expression is modulated by fasting and refeeding, and by different diets, in mice. Adipose expression of CTRP5 was markedly upregulated in obese and diabetic humans and in genetic and dietary models of obesity in rodents. Furthermore, human CTRP5 expression in the subcutaneous fat depot positively correlated with BMI. A genetic loss-of-function mouse model was used to address the metabolic function of CTRP5 in vivo. On a standard chow diet, CTRP5-deficient mice had reduced fasting insulin but were otherwise comparable with wild-type littermate controls in body weight and adiposity. However, when fed a high-fat diet, CTRP5-deficient animals had attenuated hepatic steatosis and improved insulin action. Loss of CTRP5 also improved the capacity of chow-fed aged mice to respond to subsequent high-fat feeding, as evidenced by decreased insulin resistance. In cultured adipocytes and myotubes, recombinant CTRP5 treatment attenuated insulin-stimulated Akt phosphorylation. Our results provide the first genetic and physiological evidence for CTRP5 as a negative regulator of glucose metabolism and insulin sensitivity. Inhibition of CTRP5 action may result in the alleviation of insulin resistance associated with obesity and diabetes.
Keywords: C1QTNF5; C1q/TNF-related protein 5; adipokine; diabetes; insulin sensitivity; obesity.
Copyright © 2016 the American Physiological Society.
Figures








Similar articles
-
C1q/TNF-related protein 6 (CTRP6) links obesity to adipose tissue inflammation and insulin resistance.J Biol Chem. 2017 Sep 8;292(36):14836-14850. doi: 10.1074/jbc.M116.766808. Epub 2017 Jul 18. J Biol Chem. 2017. PMID: 28726640 Free PMC article.
-
CTRP7 deletion attenuates obesity-linked glucose intolerance, adipose tissue inflammation, and hepatic stress.Am J Physiol Endocrinol Metab. 2017 Apr 1;312(4):E309-E325. doi: 10.1152/ajpendo.00344.2016. Epub 2017 Feb 21. Am J Physiol Endocrinol Metab. 2017. PMID: 28223291 Free PMC article.
-
Adipocyte C1QTNF5 expression is BMI-dependently related to early adipose tissue dysfunction and systemic CTRP5 serum levels in obese children.Int J Obes (Lond). 2017 Jun;41(6):955-963. doi: 10.1038/ijo.2017.54. Epub 2016 Dec 5. Int J Obes (Lond). 2017. PMID: 28239164
-
Late-Onset Retinal Degeneration: Clinical Features and C1QTNF5/CTRP5 Function.Adv Exp Med Biol. 2025;1468:511-516. doi: 10.1007/978-3-031-76550-6_83. Adv Exp Med Biol. 2025. PMID: 39930246 Review.
-
The role of collagen homeostasis in the pathogenesis of vascular disease associated to insulin resistance.Diabetes Metab Syndr. 2019 May-Jun;13(3):1877-1883. doi: 10.1016/j.dsx.2019.04.019. Epub 2019 Apr 17. Diabetes Metab Syndr. 2019. PMID: 31235109 Review. No abstract available.
Cited by
-
Association of decreased C1q/tumor necrosis factor-related protein-5 levels with metabolic and hormonal disturbance in polycystic ovary syndrome.J Turk Ger Gynecol Assoc. 2019 May 28;20(2):89-96. doi: 10.4274/jtgga.galenos.2018.2018.0027. Epub 2018 Jul 2. J Turk Ger Gynecol Assoc. 2019. PMID: 29964236 Free PMC article.
-
C1q/TNF-related protein 6 (CTRP6) links obesity to adipose tissue inflammation and insulin resistance.J Biol Chem. 2017 Sep 8;292(36):14836-14850. doi: 10.1074/jbc.M116.766808. Epub 2017 Jul 18. J Biol Chem. 2017. PMID: 28726640 Free PMC article.
-
Late-onset renal hypertrophy and dysfunction in mice lacking CTRP1.FASEB J. 2020 Feb;34(2):2657-2676. doi: 10.1096/fj.201900558RR. Epub 2019 Dec 26. FASEB J. 2020. PMID: 31908037 Free PMC article.
-
Comprehensive characterization of pathogenic missense CTRP6 variants and their association with cancer.BMC Cancer. 2025 Feb 20;25(1):304. doi: 10.1186/s12885-025-13685-0. BMC Cancer. 2025. PMID: 39979869 Free PMC article.
-
Adiponectin and related C1q/TNF-related proteins bind selectively to anionic phospholipids and sphingolipids.Proc Natl Acad Sci U S A. 2020 Jul 21;117(29):17381-17388. doi: 10.1073/pnas.1922270117. Epub 2020 Jul 6. Proc Natl Acad Sci U S A. 2020. PMID: 32632018 Free PMC article.
References
-
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15: 539–553, 1998. - PubMed
-
- Ayyagari R, Mandal MN, Karoukis AJ, Chen L, McLaren NC, Lichter M, Wong DT, Hitchcock PF, Caruso RC, Moroi SE, Maumenee IH, Sieving PA. Late-onset macular degeneration and long anterior lens zonules result from a CTRP5 gene mutation. Invest Ophthalmol Vis Sci 46: 3363–3371, 2005. - PubMed
-
- Bonora E, Manicardi V, Zavaroni I, Coscelli C, Butturini U. Relationships between insulin secretion, insulin metabolism and insulin resistance in mild glucose intolerance. Diabete Metab 13: 116–121, 1987. - PubMed
-
- Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 7: 95–96, 2008. - PubMed
-
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

