Using Drosophila as an integrated model to study mild repetitive traumatic brain injury
- PMID: 27143646
- PMCID: PMC4855207
- DOI: 10.1038/srep25252
Using Drosophila as an integrated model to study mild repetitive traumatic brain injury
Abstract
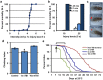
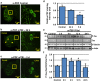
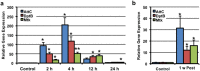
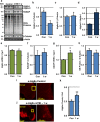
Traumatic brain injury (TBI) is a major cause of morbidity and mortality worldwide. In addition, there has been a growing appreciation that even repetitive, milder forms of TBI (mTBI) can have long-term deleterious consequences to neural tissues. Hampering our understanding of genetic and environmental factors that influence the cellular and molecular responses to injury has been the limited availability of effective genetic model systems that could be used to identify the key genes and pathways that modulate both the acute and long-term responses to TBI. Here we report the development of a severe and mild-repetitive TBI model using Drosophila. Using this system, key features that are typically found in mammalian TBI models were also identified in flies, including the activation of inflammatory and autophagy responses, increased Tau phosphorylation and neuronal defects that impair sleep-related behaviors. This novel injury paradigm demonstrates the utility of Drosophila as an effective tool to validate genetic and environmental factors that influence the whole animal response to trauma and to identify prospective therapies needed for the treatment of TBI.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

