Prolyl-4 Hydroxylase 2 (PHD2) Deficiency in Endothelial Cells and Hematopoietic Cells Induces Obliterative Vascular Remodeling and Severe Pulmonary Arterial Hypertension in Mice and Humans Through Hypoxia-Inducible Factor-2α
- PMID: 27143681
- PMCID: PMC4907810
- DOI: 10.1161/CIRCULATIONAHA.116.021494
Prolyl-4 Hydroxylase 2 (PHD2) Deficiency in Endothelial Cells and Hematopoietic Cells Induces Obliterative Vascular Remodeling and Severe Pulmonary Arterial Hypertension in Mice and Humans Through Hypoxia-Inducible Factor-2α
Abstract
Background: Vascular occlusion and complex plexiform lesions are hallmarks of the pathology of severe pulmonary arterial hypertension (PAH) in patients. However, the mechanisms of obliterative vascular remodeling remain elusive; hence, current therapies have not targeted the fundamental disease-modifying mechanisms and result in only modest improvement in morbidity and mortality.
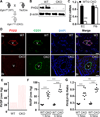
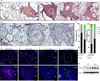
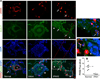
Methods and results: Mice with Tie2Cre-mediated disruption of Egln1 (encoding prolyl-4 hydroxylase 2 [PHD2]; Egln1(Tie2)) in endothelial cells and hematopoietic cells exhibited spontaneous severe PAH with extensive pulmonary vascular remodeling, including vascular occlusion and plexiform-like lesions, resembling the hallmarks of the pathology of clinical PAH. As seen in patients with idiopathic PAH, Egln1(Tie2) mice exhibited unprecedented right ventricular hypertrophy and failure and progressive mortality. Consistently, PHD2 expression was diminished in lung endothelial cells of obliterated pulmonary vessels in patients with idiopathic PAH. Genetic deletions of both Egln1 and Hif1a or Egln1 and Hif2a identified hypoxia-inducible factor-2α as the critical mediator of the severe PAH seen in Egln1(Tie2) mice. We also observed altered expression of many pulmonary hypertension-causing genes in Egln1(Tie2) lungs, which was normalized in Egln1(Tie2)/Hif2a(Tie2) lungs. PHD2-deficient endothelial cells promoted smooth muscle cell proliferation in part through hypoxia-inducible factor-2α-activated CXCL12 expression. Genetic deletion of Cxcl12 attenuated PAH in Egln1(Tie2) mice.
Conclusions: These studies defined an unexpected role of PHD2 deficiency in the mechanisms of severe PAH and identified the first genetically modified mouse model with obliterative vascular remodeling and pathophysiology recapitulating clinical PAH. Thus, targeting PHD2/hypoxia-inducible factor-2α signaling is a promising strategy to reverse vascular remodeling for treatment of severe PAH.
Keywords: endothelial cells; plexiform lesion; pulmonary heart disease; pulmonary hypertension; right heart hypertrophy and failure; vascular remodeling; vascular smooth muscle cells.
© 2016 American Heart Association, Inc.
Figures
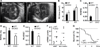



References
-
- McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, Harrington RA, Anderson JL, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Grines CL, Hlatky MA, Jacobs AK, Kaul S, Lichtenberg RC, Lindner JR, Moliterno DJ, Mukherjee D, Pohost GM, Rosenson RS, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Weitz HH, Wesley DJ ACCF/AHA. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. - PubMed
-
- Voelkel NF, Cool C. Pathology of pulmonary hypertension. Cardiol Clin. 2004;22:343–351. v. - PubMed
-
- Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder RM. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol. 2004;43:25S–32S. - PubMed
-
- Meyrick B. The pathology of pulmonary artery hypertension. Clin Chest Med. 2001;22:393–404. vii. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

