Long-term safety profile of anakinra in patients with severe cryopyrin-associated periodic syndromes
- PMID: 27143789
- PMCID: PMC4957676
- DOI: 10.1093/rheumatology/kew208
Long-term safety profile of anakinra in patients with severe cryopyrin-associated periodic syndromes
Abstract
Objective: Anakinra is approved for the treatment of RA and cryopyrin-associated periodic syndromes (CAPS). While the anakinra safety profile is well established in RA, the long-term safety profile in severe CAPS is less well documented and will therefore be discussed in this report.
Methods: A prospective, open-label, single centre, clinical cohort study was conducted at the National Institutes of Health in the USA, from 2003 to 2010, investigating the efficacy and safety of anakinra treatment for up to 5 years in 43 patients with CAPS. Safety was evaluated using adverse event (AE) reports, laboratory assessments, vital signs and diary reports.
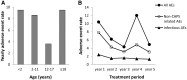
Results: In total, 1233 AEs were reported during the study, with a yearly rate of 7.7 AEs per patient. The event rate decreased over time, and dose escalation during the study did not affect AE frequency. Anakinra had similar safety profiles in adults and children. The most frequently reported AEs were typical CAPS disease symptoms such as headache and arthralgia. Injection site reactions occurred mainly during the first month of anakinra treatment. In total, 14 patients experienced 24 serious AEs (SAEs), all of which resolved during the study period. The most common types of SAEs were infections such as pneumonia and gastroenteritis. There were no permanent discontinuations of treatment due to AEs.
Conclusion: In this study anakinra treatment of patients with severe CAPS for up to 5 years was safe and well tolerated both in paediatric and adult patients, with most AEs emerging during the first months after treatment initiation.
Trial registration: ClincialTrials.gov, clinicaltrials.gov, NCT00069329.
Keywords: adverse event (AE); anakinra; clinical trial; cryopyrin-associated periodic syndromes (CAPS); headache; infections; injection site reactions (ISR); neonatal-onset multisystem inflammatory disease (NOMID); safety.
© The Author 2016. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures

Similar articles
-
Development and effect of antibodies to anakinra during treatment of severe CAPS: sub-analysis of a long-term safety and efficacy study.Clin Rheumatol. 2018 Dec;37(12):3381-3386. doi: 10.1007/s10067-018-4196-x. Epub 2018 Jul 7. Clin Rheumatol. 2018. PMID: 29982913
-
Efficacy and safety of anakinra therapy in pediatric and adult patients with the autoinflammatory Muckle-Wells syndrome.Arthritis Rheum. 2011 Mar;63(3):840-9. doi: 10.1002/art.30149. Arthritis Rheum. 2011. PMID: 21360513
-
Anakinra use during pregnancy in patients with cryopyrin-associated periodic syndromes (CAPS).Arthritis Rheumatol. 2014 Nov;66(11):3227-32. doi: 10.1002/art.38811. Arthritis Rheumatol. 2014. PMID: 25223501 Free PMC article.
-
Anakinra for the treatment of rheumatoid arthritis: a safety evaluation.Expert Opin Drug Saf. 2018 Jul;17(7):727-732. doi: 10.1080/14740338.2018.1486819. Epub 2018 Jun 14. Expert Opin Drug Saf. 2018. PMID: 29883212 Review.
-
Pharmacological treatment options for cryopyrin-associated periodic syndromes.Expert Rev Clin Pharmacol. 2017 Aug;10(8):855-864. doi: 10.1080/17512433.2017.1338946. Epub 2017 Jun 20. Expert Rev Clin Pharmacol. 2017. PMID: 28586272 Review.
Cited by
-
Human Autoinflammatory Diseases Mediated by NLRP3-, Pyrin-, NLRP1-, and NLRC4-Inflammasome Dysregulation Updates on Diagnosis, Treatment, and the Respective Roles of IL-1 and IL-18.Front Immunol. 2020 Aug 25;11:1840. doi: 10.3389/fimmu.2020.01840. eCollection 2020. Front Immunol. 2020. PMID: 32983099 Free PMC article. Review.
-
Pro-inflammatory cytokine responses to Naegleria fowleri infection.Front Trop Dis. 2022;3:1082334. doi: 10.3389/fitd.2022.1082334. Front Trop Dis. 2022. PMID: 37065537 Free PMC article.
-
Muckle-Wells syndrome: clinical perspectives.Open Access Rheumatol. 2017 Jul 11;9:123-129. doi: 10.2147/OARRR.S114447. eCollection 2017. Open Access Rheumatol. 2017. PMID: 28744167 Free PMC article. Review.
-
Inflammasome components as new therapeutic targets in inflammatory disease.Nat Rev Immunol. 2025 Jan;25(1):22-41. doi: 10.1038/s41577-024-01075-9. Epub 2024 Sep 9. Nat Rev Immunol. 2025. PMID: 39251813 Review.
-
IL-1 receptor antagonism reveals a yin-yang relationship between NFκB and interferon signaling in chronic lymphocytic leukemia.Proc Natl Acad Sci U S A. 2024 Aug 13;121(33):e2405644121. doi: 10.1073/pnas.2405644121. Epub 2024 Aug 9. Proc Natl Acad Sci U S A. 2024. PMID: 39121163 Free PMC article. Clinical Trial.
References
-
- Hoffman HM. Rilonacept for the treatment of cryopyrin-associated periodic syndromes (CAPS). Expert Opin Biol Ther 2009;9:519–31. - PubMed
-
- Lachmann HJ, Quartier P, So A, Hawkins PN. The emerging role of interleukin-1beta in autoinflammatory diseases. Arthritis Rheum 2011;63:314–24. - PubMed
-
- Touitou I, Sarkisian T, Medlej-Hashim M. et al. Country as the primary risk factor for renal amyloidosis in familial Mediterranean fever. Arthritis Rheum 2007;56:1706–12. - PubMed
-
- Prieur AM, Griscelli C, Lampert F. et al. A chronic, infantile, neurological, cutaneous and articular (CINCA) syndrome. A specific entity analysed in 30 patients. Scand J Rheumatol Suppl 1987;66:57–68. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

