Long-term efficacy and safety of oxycodone-naloxone prolonged release in geriatric patients with moderate-to-severe chronic noncancer pain: a 52-week open-label extension phase study
- PMID: 27143857
- PMCID: PMC4844303
- DOI: 10.2147/DDDT.S106025
Long-term efficacy and safety of oxycodone-naloxone prolonged release in geriatric patients with moderate-to-severe chronic noncancer pain: a 52-week open-label extension phase study
Abstract
Background: Two-thirds of older people suffer from chronic pain and finding valid treatment options is essential. In this 1-yearlong investigation, we evaluated the efficacy and safety of prolonged-release oxycodone-naloxone (OXN-PR) in patients aged ≥70 (mean 81.7) years.
Methods: In this open-label prospective study, patients with moderate-to-severe noncancer chronic pain were prescribed OXN-PR for 1 year. The primary endpoint was the proportion of patients who achieved ≥30% reduction in pain intensity after 52 weeks of treatment, without worsening bowel function. The scheduled visits were at baseline (T0), after 4 weeks (T4), and after 52 weeks (T52).
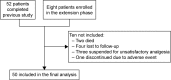
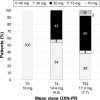
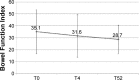
Results: Fifty patients completed the study. The primary endpoint was achieved in 78% of patients at T4 and 96% at T52 (P<0.0001). Pain intensity, measured on a 0-10 numerical rating scale, decreased from 6.0 at T0 to 2.8 at T4 and to 1.7 at T52 (P<0.0001). Mean daily dose of oxycodone increased from 10 to 14.4 mg (T4) and finally to 17.4 mg (T52). Bowel Function Index from 35.1 to 28.7 at T52. No changes were observed in cognitive functions (Mini-Mental State Examination evaluation), while daily functioning improved (Barthel Index from 53.1 to 61.0, P<0.0001). The Screener and Opioid Assessment for Patients with Pain-Revised score at 52 weeks was 2.6 (standard deviation 1.6), indicating a low risk of aberrant medication-related behavior. In general, OXN-PR was well tolerated.
Conclusion: This study of the long-term treatment of chronic pain in a geriatric population with OXN-PR shows satisfying analgesic effects achieved with a stable low daily dose, coupled with a good safety profile and, in particular, with a reduction of constipation, often present during opioid therapy. Our findings support the indications of the American Geriatrics Society, suggesting the use of opioids to treat pain in older people not responsive to acetaminophen or nonsteroidal anti-inflammatory drugs.
Keywords: chronic noncancer pain; constipation; geriatric; oxycodone/naloxone.
Figures


Similar articles
-
Switching to low-dose oral prolonged-release oxycodone/naloxone from WHO-Step I drugs in elderly patients with chronic pain at high risk of early opioid discontinuation.Clin Interv Aging. 2016 May 13;11:641-9. doi: 10.2147/CIA.S105821. eCollection 2016. Clin Interv Aging. 2016. PMID: 27257377 Free PMC article.
-
Efficacy and tolerability of low-dose oral prolonged-release oxycodone/naloxone for chronic nononcological pain in older patients.Clin Interv Aging. 2014 Dec 16;10:1-11. doi: 10.2147/CIA.S72521. eCollection 2015. Clin Interv Aging. 2014. PMID: 25565782 Free PMC article.
-
High dosage of a fixed combination oxycodone/naloxone prolonged release: efficacy and tolerability in patients with chronic cancer pain.Support Care Cancer. 2017 Oct;25(10):3051-3058. doi: 10.1007/s00520-017-3709-5. Epub 2017 May 3. Support Care Cancer. 2017. PMID: 28470370 Clinical Trial.
-
Quality of life and healthcare resource in patients receiving opioids for chronic pain: a review of the place of oxycodone/naloxone.Clin Drug Investig. 2015 Jan;35(1):1-11. doi: 10.1007/s40261-014-0254-6. Clin Drug Investig. 2015. PMID: 25479959 Free PMC article. Review.
-
Oral Prolonged-Release Oxycodone/Naloxone for Managing Pain and Opioid-Induced Constipation: A Review of the Evidence.Pain Pract. 2018 Jun;18(5):647-665. doi: 10.1111/papr.12646. Epub 2017 Nov 28. Pain Pract. 2018. PMID: 28944983 Review.
Cited by
-
Efficacy and tolerability of tapentadol for the treatment of chronic low back pain in elderly patients.Aging Clin Exp Res. 2021 Apr;33(4):973-982. doi: 10.1007/s40520-020-01586-0. Epub 2020 May 16. Aging Clin Exp Res. 2021. PMID: 32418129
-
Insights into the Current and Possible Future Use of Opioid Antagonists in Relation to Opioid-Induced Constipation and Dysbiosis.Molecules. 2023 Nov 24;28(23):7766. doi: 10.3390/molecules28237766. Molecules. 2023. PMID: 38067494 Free PMC article. Review.
-
New opioid prescribing guidelines released in the US: what impact will they have in the care of older patients with persistent pain?Curr Med Res Opin. 2017 Feb;33(2):275-278. doi: 10.1080/03007995.2016.1254603. Epub 2016 Nov 10. Curr Med Res Opin. 2017. PMID: 27786538 Free PMC article. No abstract available.
-
The effect of opioids on the cognitive function of older adults: results from the Personality and Total Health through life study.Age Ageing. 2021 Sep 11;50(5):1699-1708. doi: 10.1093/ageing/afab048. Age Ageing. 2021. PMID: 33755047 Free PMC article.
-
Cognitive Function During Opioid Tapering in Patients with Chronic Pain: A Prospective Cohort Study.J Pain Res. 2020 Dec 14;13:3385-3394. doi: 10.2147/JPR.S273025. eCollection 2020. J Pain Res. 2020. PMID: 33363405 Free PMC article.
References
-
- World Health Organization . Cancer Pain Relief. 2nd ed. Geneva: World Health Organization; 1996.
-
- American Geriatrics Society Panel on Chronic Pain in Older Persons The management of chronic pain in older persons: AGS Panel on chronic pain in older persons. J Am Geriatr Soc. 1998;46(5):635–651. - PubMed
-
- Abdulla A, Adams N, Bone M, et al. Guidance on the management of pain in older people. Age Ageing. 2013;42(Suppl 1):i1–i57. - PubMed
-
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331–1346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

