Potential antitumor activity of novel DODAC/PHO-S liposomes
- PMID: 27143880
- PMCID: PMC4841408
- DOI: 10.2147/IJN.S90850
Potential antitumor activity of novel DODAC/PHO-S liposomes
Abstract
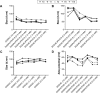
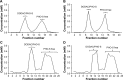
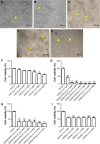
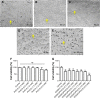
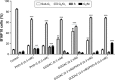
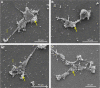
In recent studies, we showed that synthetic phosphoethanolamine (PHO-S) has a great potential for inducing cell death in several tumor cell lines without damage to normal cells. However, its cytotoxic effect and selectivity against tumor cells could increase with encapsulation in cationic liposomes, such as dioctadecyldimethylammonium chloride (DODAC), due to electrostatic interactions between these liposomes and tumor cell membranes. Our aim was to use cationic liposomes to deliver PHO-S and to furthermore maximize the therapeutic effect of this compound. DODAC liposomes containing PHO-S (DODAC/PHO-S), at concentrations of 0.3-2.0 mM, prepared by ultrasonication, were analyzed by scanning electron microscopy (SEM) and dynamic light scattering. The cytotoxic effect of DODAC/PHO-S on B16F10 cells, Hepa1c1c7 cells, and human umbilical vein endothelial cells (HUVECs) was assessed by MTT assay. Cell cycle phases of B16F10 cells were analyzed by flow cytometry and the morphological changes by SEM, after treatment. The liposomes were spherical and polydisperse in solution. The liposomes were stable, presenting an average of ∼ 50% of PHO-S encapsulation, with a small reduction after 40 days. DODAC demonstrated efficient PHO-S delivery, with the lowest values of IC50% (concentration that inhibits 50% of the growth of cells) for tumor cells, compared with PHO-S alone, with an IC50% value of 0.8 mM for B16F10 cells and 0.2 mM for Hepa1c1c7 cells, and without significant effects on endothelial cells. The Hepa1c1c7 cells showed greater sensitivity to the DODAC/PHO-S formulation when compared to B16F10 cells and HUVECs. The use of DODAC/PHO-S on B16F10 cells induced G2/M-phase cell cycle arrest, with the proportion significantly greater than that treated with PHO-S alone. The morphological analysis of B16F10 cells by SEM showed changes such as "bleb" formation, cell detachment, cytoplasmic retraction, and apoptotic bodies after DODAC/PHO-S treatment. Cationic liposomal formulation for PHO-S delivery promoted cytotoxicity more selectively and effectively against B16F10 and Hepa1c1c7 cells. Thus, the DODAC/PHO-S liposomal formulation presents great potential for preclinical studies.
Keywords: hepatocellular carcinoma; lipossomal formulation; nanocarriers; synthetic phosphoethanolamine.
Figures





References
-
- Ríos-Marco P, Jiménez-López JM, Marco C, Segovia JL, Carrasco MP. Antitumoral alkylphospholipids induce cholesterol efflux from the plasma membrane in HepG2 cells. J Pharmacol Exp Ther. 2011;336(3):866–873. - PubMed
-
- Van Blitterswijk WJ, Verheij M. Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects. Curr Pharm Des. 2008;14(21):2061–2074. - PubMed
-
- Van Blitterswijk WJ, Verheij M. Anticancer mechanisms and clinical application of alkylphospholipids. Biochim Biophys Acta. 2013;1831(3):663–674. - PubMed
-
- Ferreira AK, Meneguelo R, Neto SC, Chierice GO, Maria DA. Synthetic phosphoethanolamine induces apoptosis through caspase-3 pathway by decreasing expression of bax/bad protein and changes cell cycle in melanoma. J Cancer Sci Ther. 2011;3(1):53–59.
-
- Ferreira AK, Meneguelo R, Marques FL, et al. Synthetic phosphoethanolamine a precursor of membrane phospholipids reduce tumor growth in mice bearing melanoma B16-F10 and in vitro induce apoptosis and arrest in G2/M phase. Biomed Pharmacother. 2012;66(7):541–548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

