Establishment and validation of a prognostic nomogram for patients with resectable perihilar cholangiocarcinoma
- PMID: 27144432
- PMCID: PMC5095079
- DOI: 10.18632/oncotarget.9104
Establishment and validation of a prognostic nomogram for patients with resectable perihilar cholangiocarcinoma
Abstract
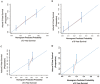
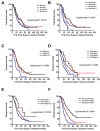
As the conventional staging systems have poor prognosis prediction ability for patients with perihilar cholangiocarcinoma (pCCA), we established and validated an effective prognostic nomogram for pCCA patients based on their personal and tumor characteristics. A total of 235 patients who received curative intent resections at the Eastern Hepatobiliary Surgery Hospital from 2000 to 2009 were recruited as the primary training cohort. Age, preoperative CA19-9 levels, portal vein involvement, hepatic artery invasion, lymph node metastases, and surgical treatment outcomes (R0 or R1/2) were independent prognostic factors for pCCA patients in the primary cohort as suggested by the multivariate analyses and these were included in the established nomogram. The calibration curve showed good agreement between overall survival probability of pCCA patients for the nomogram predictions and the actual observations and the concordance index (C-index) was 0.68 (95% CI, 0.61-0.71). The C-index values and time-dependent ROC tests suggested that the nomogram is superior to the conventional staging systems including the Bismuth-Corlette, Gazzaniga, Memorial Sloan Kettering Cancer Center (MSKCC), American Joint Committee on Cancer (AJCC) TNM 7th edition, and Mayo Clinic. The nomogram also performed better than the traditional staging system in the internal cohort with 93 pCCA patients from the same institution and an external validation cohort including 84 pCCA patients from another institution in predicting the overall survival of the pCCA patients as suggested by the C-index values and the time-dependent ROC tests. In summary, the proposed nomogram has superior predictive accuracy of prognosis for resectable pCCA patients.
Keywords: nomogram; overall survival; perihilar cholangiocarcinoma.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures

References
-
- Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Wasan H, British Society of G Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657–1669. - PubMed
-
- Williams TM, Majithia L, Wang SJ, Thomas CR., Jr Defining the role of adjuvant therapy: cholangiocarcinoma and gall bladder cancer. Semin Radiat Oncol. 2014;24:94–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

