MEF2C-MYOCD and Leiomodin1 Suppression by miRNA-214 Promotes Smooth Muscle Cell Phenotype Switching in Pulmonary Arterial Hypertension
- PMID: 27144530
- PMCID: PMC4856285
- DOI: 10.1371/journal.pone.0153780
MEF2C-MYOCD and Leiomodin1 Suppression by miRNA-214 Promotes Smooth Muscle Cell Phenotype Switching in Pulmonary Arterial Hypertension
Abstract
Background: Vascular hyperproliferative disorders are characterized by excessive smooth muscle cell (SMC) proliferation leading to vessel remodeling and occlusion. In pulmonary arterial hypertension (PAH), SMC phenotype switching from a terminally differentiated contractile to synthetic state is gaining traction as our understanding of the disease progression improves. While maintenance of SMC contractile phenotype is reportedly orchestrated by a MEF2C-myocardin (MYOCD) interplay, little is known regarding molecular control at this nexus. Moreover, the burgeoning interest in microRNAs (miRs) provides the basis for exploring their modulation of MEF2C-MYOCD signaling, and in turn, a pro-proliferative, synthetic SMC phenotype. We hypothesized that suppression of SMC contractile phenotype in pulmonary hypertension is mediated by miR-214 via repression of the MEF2C-MYOCD-leiomodin1 (LMOD1) signaling axis.
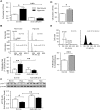
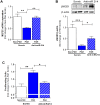
Methods and results: In SMCs isolated from a PAH patient cohort and commercially obtained hPASMCs exposed to hypoxia, miR-214 expression was monitored by qRT-PCR. miR-214 was upregulated in PAH- vs. control subject hPASMCs as well as in commercially obtained hPASMCs exposed to hypoxia. These increases in miR-214 were paralleled by MEF2C, MYOCD and SMC contractile protein downregulation. Of these, LMOD1 and MEF2C were directly targeted by the miR. Mir-214 overexpression mimicked the PAH profile, downregulating MEF2C and LMOD1. AntagomiR-214 abrogated hypoxia-induced suppression of the contractile phenotype and its attendant proliferation. Anti-miR-214 also restored PAH-PASMCs to a contractile phenotype seen during vascular homeostasis.
Conclusions: Our findings illustrate a key role for miR-214 in modulation of MEF2C-MYOCD-LMOD1 signaling and suggest that an antagonist of miR-214 could mitigate SMC phenotype changes and proliferation in vascular hyperproliferative disorders including PAH.
Conflict of interest statement
Figures






References
-
- Stenmark KR, Fagan KA, Frid MG (2006) Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691. - PubMed
-
- Mandegar M, Fung YC, Huang W, Remillard CV, Rubin LJ, Yuan JX (2004) Cellular and molecular mechanisms of pulmonary vascular remodeling: role in the development of pulmonary hypertension. Microvasc Res 68: 75–103. - PubMed
-
- Rabinovitch M (2007) Pathobiology of pulmonary hypertension. Annu Rev Pathol 2: 369–399. - PubMed
-
- Lanner MC, Raper M, Pratt WM, Rhoades RA (2005) Heterotrimeric G proteins and the platelet-derived growth factor receptor-beta contribute to hypoxic proliferation of smooth muscle cells. Am J Respir Cell Mol Biol 33: 412–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

