Exogenous and Endogeneous Disialosyl Ganglioside GD1b Induces Apoptosis of MCF-7 Human Breast Cancer Cells
- PMID: 27144558
- PMCID: PMC4881478
- DOI: 10.3390/ijms17050652
Exogenous and Endogeneous Disialosyl Ganglioside GD1b Induces Apoptosis of MCF-7 Human Breast Cancer Cells
Abstract
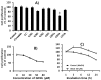
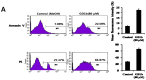
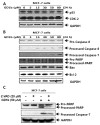
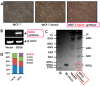
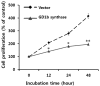
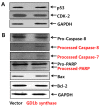
Gangliosides have been known to play a role in the regulation of apoptosis in cancer cells. This study has employed disialyl-ganglioside GD1b to apoptosis in human breast cancer MCF-7 cells using exogenous treatment of the cells with GD1b and endogenous expression of GD1b in MCF-7 cells. First, apoptosis in MCF-7 cells was observed after treatment of GD1b. Treatment of MCF-7 cells with GD1b reduced cell growth rates in a dose and time dependent manner during GD1b treatment, as determined by XTT assay. Among the various gangliosides, GD1b specifically induced apoptosis of the MCF-7 cells. Flow cytometry and immunofluorescence assays showed that GD1b specifically induces apoptosis in the MCF-7 cells with Annexin V binding for apoptotic actions in early stage and propidium iodide (PI) staining the nucleus of the MCF-7 cells. Treatment of MCF-7 cells with GD1b activated apoptotic molecules such as processed forms of caspase-8, -7 and PARP (Poly(ADP-ribose) polymerase), without any change in the expression of mitochondria-mediated apoptosis molecules such as Bax and Bcl-2. Second, to investigate the effect of endogenously produced GD1b on the regulation of cell function, UDP-gal: β1,3-galactosyltransferase-2 (GD1b synthase, Gal-T2) gene has been transfected into the MCF-7 cells. Using the GD1b synthase-transfectants, apoptosis-related signal proteins linked to phenotype changes were examined. Similar to the exogenous GD1b treatment, the cell growth of the GD1b synthase gene-transfectants was significantly suppressed compared with the vector-transfectant cell lines and transfection activated the apoptotic molecules such as processed forms of caspase-8, -7 and PARP, but not the levels of expression of Bax and Bcl-2. GD1b-induced apoptosis was blocked by caspase inhibitor, Z-VAD. Therefore, taken together, it was concluded that GD1b could play an important role in the regulation of breast cancer apoptosis.
Keywords: Gal-T2); apoptosis; caspase; disialyl-ganglioside GD1b; human breast cancer MCF-7 cells; human β1,3-galactosyltransferase-2 (GD1b synthase.
Figures


Similar articles
-
Progressive resistance to apoptosis in a cell lineage model of human proliferative breast disease.J Natl Cancer Inst. 2001 May 16;93(10):776-82. doi: 10.1093/jnci/93.10.776. J Natl Cancer Inst. 2001. PMID: 11353788
-
P53-mediated cell cycle arrest and apoptosis through a caspase-3- independent, but caspase-9-dependent pathway in oridonin-treated MCF-7 human breast cancer cells.Acta Pharmacol Sin. 2007 Jul;28(7):1057-66. doi: 10.1111/j.1745-7254.2007.00588.x. Acta Pharmacol Sin. 2007. PMID: 17588343
-
RRR-alpha-tocopheryl succinate-induced apoptosis of human breast cancer cells involves Bax translocation to mitochondria.Cancer Res. 2003 May 15;63(10):2483-91. Cancer Res. 2003. PMID: 12750270
-
Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation.J Natl Cancer Inst. 2005 Dec 7;97(23):1746-59. doi: 10.1093/jnci/dji400. J Natl Cancer Inst. 2005. PMID: 16333030
-
Heregulin-induced apoptosis is mediated by down-regulation of Bcl-2 and activation of caspase-7 and is potentiated by impairment of protein kinase C alpha activity.Oncogene. 2001 Dec 13;20(57):8258-69. doi: 10.1038/sj.onc.1205039. Oncogene. 2001. PMID: 11781840
Cited by
-
Biological Roles of Aberrantly Expressed Glycosphingolipids and Related Enzymes in Human Cancer Development and Progression.Front Physiol. 2018 May 3;9:466. doi: 10.3389/fphys.2018.00466. eCollection 2018. Front Physiol. 2018. PMID: 29773994 Free PMC article. Review.
-
The Ying and Yang of Ganglioside Function in Cancer.Cancers (Basel). 2023 Nov 10;15(22):5362. doi: 10.3390/cancers15225362. Cancers (Basel). 2023. PMID: 38001622 Free PMC article. Review.
-
Disialyl GD2 ganglioside suppresses ICAM-1-mediated invasiveness in human breast cancer MDA-MB231 cells.Int J Biol Sci. 2017 Feb 12;13(3):265-275. doi: 10.7150/ijbs.16903. eCollection 2017. Int J Biol Sci. 2017. PMID: 28367091 Free PMC article.
-
Deciphering the Importance of Glycosphingolipids on Cellular and Molecular Mechanisms Associated with Epithelial-to-Mesenchymal Transition in Cancer.Biomolecules. 2021 Jan 6;11(1):62. doi: 10.3390/biom11010062. Biomolecules. 2021. PMID: 33418847 Free PMC article. Review.
-
Sphingolipids in spinal cord injury.Int J Physiol Pathophysiol Pharmacol. 2016 Aug 5;8(2):52-69. eCollection 2016. Int J Physiol Pathophysiol Pharmacol. 2016. PMID: 27570580 Free PMC article. Review.
References
-
- Choi H.J., Chung T.W., Kang S.K., Lee Y.C., Ko J.H., Kim J.G., Kim C.H. Ganglioside GM3 modulates tumor suppressor PTEN-mediated cell cycle progression—Transcriptional induction of p21(WAF1) and p27(kip1) by inhibition of PI-3K/AKT pathway. Glycobiology. 2006;16:573–583. doi: 10.1093/glycob/cwj105. - DOI - PubMed
-
- Kang N.Y., Kang S.K., Lee Y.C., Choi H.J., Lee Y.S., Cho S.Y., Kim Y.S., Ko J.H., Kim C.H. Transcriptional regulation of the human GD3 synthase gene expression in Fas-induced Jurkat T cells: A critical role of transcription factor NF-kappaB in regulated expression. Glycobiology. 2006;16:375–389. doi: 10.1093/glycob/cwj087. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

