Quantitative Real-Time Polymerase Chain Reaction Measurement of HLA-DRA Gene Expression in Whole Blood Is Highly Reproducible and Shows Changes That Reflect Dynamic Shifts in Monocyte Surface HLA-DR Expression during the Course of Sepsis
- PMID: 27144640
- PMCID: PMC4856385
- DOI: 10.1371/journal.pone.0154690
Quantitative Real-Time Polymerase Chain Reaction Measurement of HLA-DRA Gene Expression in Whole Blood Is Highly Reproducible and Shows Changes That Reflect Dynamic Shifts in Monocyte Surface HLA-DR Expression during the Course of Sepsis
Abstract
Introduction: A decrease in the expression of monocyte surface protein HLA-DR (mHLA-DR), measured by flow cytometry (FCM), has been suggested as a marker of immunosuppression and negative outcome in severe sepsis. However, FCM is not always available due to sample preparation that limits its use to laboratory operational hours. In this prospective study we evaluated dynamic changes in mHLA-DR expression during sepsis in relation to changes in HLA-DRA gene expression and Class II transactivator (CIITA), measured by quantitative Real-Time Polymerase Chain Reaction (qRT-PCR).
Aims: The aims of this study were: 1. to validate the robustness of qRT-PCR measurement of HLA-DRA- and CIITA-mRNA expression, in terms of reproducibility; and 2. to see if changes in expression of these genes reflect changes in mHLA-DR expression during the course of severe and non-severe bacteraemic sepsis.
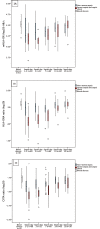
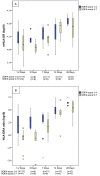
Methods and findings: Blood samples were collected from 60 patients with bacteraemic sepsis on up to five occasions during Days 1-28 after hospital admission. We found the reproducibility of the qRT-PCR method to be high by demonstrating low threshold variations (<0.11 standard deviation (SD)) of the qRT-PCR system, low intra-assay variation of Ct-values within triplicates (≤0.15 SD) and low inter-assay variations (12%) of the calculated target gene ratios. Our results also revealed dynamic HLA-DRA expression patterns during the course of sepsis that reflected those of mHLA-DR measured by FCM. Furthermore, HLA-DRA and mHLA-DR recovery slopes in patients with non-severe sepsis differed from those in patients with severe sepsis, shown by mixed model for repeated measurements (p<0.05). However, during the first seven days of sepsis, PCR-measurements showed a higher magnitude of difference between the two sepsis groups. Mean differences (95% CI) between severe sepsis (n = 20) and non-severe sepsis (n = 40) were; on day 1-2, HLA-DRA 0.40 (0.28-0.59) p<0.001, CIITA 0.48 (0.32-0.72) p = 0.005, mHLA-DR 0.63 (0.45-1.00) p = 0.04, day 7 HLA-DRA 0.59 (0.46-0.77) p<0.001, CIITA 0.56 (0.41-0.76) p<0.001, mHLA-DR 0.81 (0.66-1.00) p = 0.28.
Conclusion: We conclude that qRT-PCR measurement of HLA-DRA expression is robust, and that this method appears to be preferable to FCM in identifying patients with severe sepsis that may benefit from immunostimulation.
Conflict of interest statement
Figures
Similar articles
-
Utility of monocyte HLA-DR and rationale for therapeutic GM-CSF in sepsis immunoparalysis.Front Immunol. 2023 Feb 7;14:1130214. doi: 10.3389/fimmu.2023.1130214. eCollection 2023. Front Immunol. 2023. PMID: 36825018 Free PMC article. Review.
-
Expression of HLA-DRA and CD74 mRNA in whole blood during the course of complicated and uncomplicated Staphylococcus aureus bacteremia.Microbiol Immunol. 2017 Oct;61(10):442-451. doi: 10.1111/1348-0421.12533. Microbiol Immunol. 2017. PMID: 28862321
-
Preliminary results in quantitation of HLA-DRA by real-time PCR: a promising approach to identify immunosuppression in sepsis.Crit Care. 2013 Oct 6;17(5):R223. doi: 10.1186/cc13046. Crit Care. 2013. PMID: 24093602 Free PMC article.
-
Human leucocyte antigen (HLA-DR) gene expression is reduced in sepsis and correlates with impaired TNFα response: A diagnostic tool for immunosuppression?PLoS One. 2017 Aug 3;12(8):e0182427. doi: 10.1371/journal.pone.0182427. eCollection 2017. PLoS One. 2017. PMID: 28771573 Free PMC article.
-
Dynamic monitoring of monocyte HLA-DR expression for the diagnosis, prognosis, and prediction of sepsis.Front Biosci (Landmark Ed). 2017 Mar 1;22(8):1344-1354. doi: 10.2741/4547. Front Biosci (Landmark Ed). 2017. PMID: 28199206 Review.
Cited by
-
Cell-Type-Specific Immune Dysregulation in Severely Ill COVID-19 Patients.Cell Rep. 2021 Jan 5;34(1):108590. doi: 10.1016/j.celrep.2020.108590. Epub 2020 Dec 16. Cell Rep. 2021. PMID: 33357411 Free PMC article.
-
Inflammatory Response and Anti-Inflammatory Treatment in Persistent Inflammation-Immunosuppression-Catabolism Syndrome (PICS).J Inflamm Res. 2025 Feb 14;18:2267-2281. doi: 10.2147/JIR.S504694. eCollection 2025. J Inflamm Res. 2025. PMID: 39968098 Free PMC article. Review.
-
Plasma Transfusion Promoted Reprogramming CD4+ T Lymphocytes Immune Response in Severe Sepsis Mice Model Through Modulating the Exosome Protein Galectin 9.Cell Transplant. 2020 Jan-Dec;29:963689720947347. doi: 10.1177/0963689720947347. Cell Transplant. 2020. PMID: 32907380 Free PMC article.
-
Utility of monocyte HLA-DR and rationale for therapeutic GM-CSF in sepsis immunoparalysis.Front Immunol. 2023 Feb 7;14:1130214. doi: 10.3389/fimmu.2023.1130214. eCollection 2023. Front Immunol. 2023. PMID: 36825018 Free PMC article. Review.
-
Immunomonitoring of Monocyte and Neutrophil Function in Critically Ill Patients: From Sepsis and/or Trauma to COVID-19.J Clin Med. 2021 Dec 12;10(24):5815. doi: 10.3390/jcm10245815. J Clin Med. 2021. PMID: 34945111 Free PMC article. Review.
References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical Care Medicine 29: 1303–1310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

