Self-organization of the human embryo in the absence of maternal tissues
- PMID: 27144686
- PMCID: PMC5049689
- DOI: 10.1038/ncb3347
Self-organization of the human embryo in the absence of maternal tissues
Abstract
Remodelling of the human embryo at implantation is indispensable for successful pregnancy. Yet it has remained mysterious because of the experimental hurdles that beset the study of this developmental phase. Here, we establish an in vitro system to culture human embryos through implantation stages in the absence of maternal tissues and reveal the key events of early human morphogenesis. These include segregation of the pluripotent embryonic and extra-embryonic lineages, and morphogenetic rearrangements leading to generation of a bilaminar disc, formation of a pro-amniotic cavity within the embryonic lineage, appearance of the prospective yolk sac, and trophoblast differentiation. Using human embryos and human pluripotent stem cells, we show that the reorganization of the embryonic lineage is mediated by cellular polarization leading to cavity formation. Together, our results indicate that the critical remodelling events at this stage of human development are embryo-autonomous, highlighting the remarkable and unanticipated self-organizing properties of human embryos.
Figures


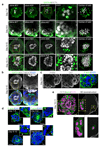
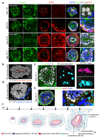
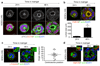
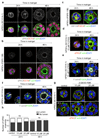
Comment in
-
The Will of the Human Embryo.Biol Reprod. 2016 Jul;95(1):2. doi: 10.1095/biolreprod.116.142364. Epub 2016 Jun 1. Biol Reprod. 2016. PMID: 27387870 No abstract available.
References
-
- Edwards RG, Bavister BD, Steptoe PC. Early stages of fertilization in vitro of human oocytes matured in vitro. Nature. 1969;221:632–635. - PubMed
-
- Edwards RG, Steptoe PC, Purdy JM. Fertilization and cleavage in vitro of preovulator human oocytes. Nature. 1970;227:1307–1309. - PubMed
-
- Koot YE, Teklenburg G, Salker MS, Brosens JJ, Macklon NS. Molecular aspects of implantation failure. Biochim Biophys Acta. 2012;1822:1943–1950. - PubMed
-
- Enders AC, Schlafke S, Hendrickx AG. Differentiation of the embryonic disc, amnion, and yolk sac in the rhesus monkey. Am J Anat. 1986;177:161–185. - PubMed
-
- Pera MF, Trounson AO. Human embryonic stem cells: prospects for development. Development. 2004;131:5515–5525. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

