A Luciferase-Expressing Leishmania braziliensis Line That Leads to Sustained Skin Lesions in BALB/c Mice and Allows Monitoring of Miltefosine Treatment Outcome
- PMID: 27144739
- PMCID: PMC4856402
- DOI: 10.1371/journal.pntd.0004660
A Luciferase-Expressing Leishmania braziliensis Line That Leads to Sustained Skin Lesions in BALB/c Mice and Allows Monitoring of Miltefosine Treatment Outcome
Abstract
Background: Leishmania braziliensis is the most prevalent species isolated from patients displaying cutaneous and muco-cutaneous leishmaniasis in South America. However, there are difficulties for studying L. braziliensis pathogenesis or response to chemotherapy in vivo due to the natural resistance of most mouse strains to infection with these parasites. The aim of this work was to develop an experimental set up that could be used to assess drug efficacy against L. braziliensis. The model was tested using miltefosine.
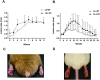
Methodology/principal findings: A L. braziliensis line, originally isolated from a cutaneous leishmaniasis patient, was passaged repeatedly in laboratory rodents and further genetically manipulated to express luciferase. Once collected from a culture of parasites freshly transformed from amastigotes, 106 wild type or luciferase-expressing stationary phase promastigotes were inoculated subcutaneously in young BALB/c mice or golden hamsters. In both groups, sustained cutaneous lesions developed at the site of inoculation, no spontaneous self- healing being observed 4 months post-inoculation, if left untreated. Compared to the wild type line features, no difference was noted for the luciferase-transgenic line. Infected animals were treated with 5 or 15 mg/kg/day miltefosine orally for 15 days. At the end of treatment, lesions had regressed and parasites were not detected. However, relapses were observed in animals treated with both doses of miltefosine.
Conclusions/significance: Here we described experimental settings for a late-healing model of cutaneous leishmaniasis upon inoculation of a luciferase-expressing L. braziliensis line that can be applied to drug development projects. These settings allowed the monitoring of the transient efficacy of a short-term miltefosine administration.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Llanos Cuentas EA, Cuba CC, Barreto AC, Marsden PD. Clinical characteristics of human Leishmania braziliensis braziliensis infections. Trans R Soc Trop Med Hyg. 1984;78(6):845–6. Epub 1984/01/01. . - PubMed
-
- Jones TC, Johnson WD Jr., Barretto AC, Lago E, Badaro R, Cerf B, et al. Epidemiology of American cutaneous leishmaniasis due to Leishmania braziliensis braziliensis. J Infect Dis. 1987;156(1):73–83. Epub 1987/07/01. . - PubMed
-
- Jirmanus L, Glesby MJ, Guimaraes LH, Lago E, Rosa ME, Machado PR, et al. Epidemiological and clinical changes in American tegumentary leishmaniasis in an area of Leishmania (Viannia) braziliensis transmission over a 20-year period. Am J Trop Med Hyg. 2012;86(3):426–33. Epub 2012/03/10. 10.4269/ajtmh.2012.11-0378 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

