CUG-BP, Elav-like family member 1 (CELF1) is required for normal myofibrillogenesis, morphogenesis, and contractile function in the embryonic heart
- PMID: 27144987
- PMCID: PMC4946975
- DOI: 10.1002/dvdy.24413
CUG-BP, Elav-like family member 1 (CELF1) is required for normal myofibrillogenesis, morphogenesis, and contractile function in the embryonic heart
Abstract
Background: CUG-BP, Elav-like family member 1 (CELF1) is a multifunctional RNA binding protein found in a variety of adult and embryonic tissues. In the heart, CELF1 is found exclusively in the myocardium. However, the roles of CELF1 during cardiac development have not been completely elucidated.
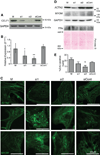
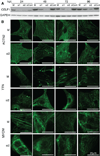
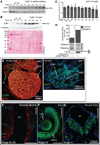
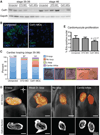
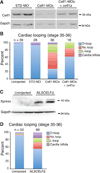
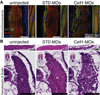
Results: Myofibrillar organization is disrupted and proliferation is reduced following knockdown of CELF1 in cultured chicken primary embryonic cardiomyocytes. In vivo knockdown of Celf1 in developing Xenopus laevis embryos resulted in myofibrillar disorganization and a trend toward reduced proliferation in heart muscle, indicating conserved roles for CELF1 orthologs in embryonic cardiomyocytes. Loss of Celf1 also resulted in morphogenetic abnormalities in the developing heart and gut. Using optical coherence tomography, we showed that cardiac contraction was impaired following depletion of Celf1, while heart rhythm remained unperturbed. In contrast to cardiac muscle, loss of Celf1 did not disrupt myofibril organization in skeletal muscle cells, although it did lead to fragmentation of skeletal muscle bundles.
Conclusions: CELF1 is required for normal myofibril organization, proliferation, morphogenesis, and contractile performance in the developing myocardium. Developmental Dynamics 245:854-873, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: CELF1; Xenopus; chicken; development; heart; myofibrillogenesis; optical coherence tomography.
© 2016 Wiley Periodicals, Inc.
Figures





Similar articles
-
Identification of Targets of CUG-BP, Elav-Like Family Member 1 (CELF1) Regulation in Embryonic Heart Muscle.PLoS One. 2016 Feb 11;11(2):e0149061. doi: 10.1371/journal.pone.0149061. eCollection 2016. PLoS One. 2016. PMID: 26866591 Free PMC article.
-
Identification of transcripts regulated by CUG-BP, Elav-like family member 1 (CELF1) in primary embryonic cardiomyocytes by RNA-seq.Genom Data. 2015 Dec 1;6:74-76. doi: 10.1016/j.gdata.2015.08.014. Genom Data. 2015. PMID: 26366374 Free PMC article.
-
Diversity and conservation of CELF1 and CELF2 RNA and protein expression patterns during embryonic development.Dev Dyn. 2013 Jun;242(6):767-77. doi: 10.1002/dvdy.23959. Epub 2013 Apr 15. Dev Dyn. 2013. PMID: 23468433 Free PMC article.
-
Increased nuclear but not cytoplasmic activities of CELF1 protein leads to muscle wasting.Hum Mol Genet. 2020 Jun 27;29(10):1729-1744. doi: 10.1093/hmg/ddaa095. Hum Mol Genet. 2020. PMID: 32412585 Free PMC article.
-
CELF1 is Up-Regulated in Glioma and Promotes Glioma Cell Proliferation by Suppression of CDKN1B.Int J Biol Sci. 2015 Sep 22;11(11):1314-24. doi: 10.7150/ijbs.11344. eCollection 2015. Int J Biol Sci. 2015. PMID: 26535026 Free PMC article.
Cited by
-
Curriculum vitae of CUG binding protein 1 (CELF1) in homeostasis and diseases: a systematic review.Cell Mol Biol Lett. 2024 Mar 5;29(1):32. doi: 10.1186/s11658-024-00556-y. Cell Mol Biol Lett. 2024. PMID: 38443798 Free PMC article.
-
Post-Transcriptional Modification by Alternative Splicing and Pathogenic Splicing Variants in Cardiovascular Development and Congenital Heart Defects.Int J Mol Sci. 2023 Jan 13;24(2):1555. doi: 10.3390/ijms24021555. Int J Mol Sci. 2023. PMID: 36675070 Free PMC article. Review.
-
Bruno-3 regulates sarcomere component expression and contributes to muscle phenotypes of myotonic dystrophy type 1.Dis Model Mech. 2018 May 21;11(5):dmm031849. doi: 10.1242/dmm.031849. Dis Model Mech. 2018. PMID: 29716962 Free PMC article.
-
Population structure and selection signal analysis of indigenous sheep from the southern edge of the Taklamakan Desert.BMC Genomics. 2024 Jul 9;25(1):681. doi: 10.1186/s12864-024-10581-y. BMC Genomics. 2024. PMID: 38982349 Free PMC article.
References
-
- Barreau C, Paillard L, Mereau A, Osborne HB. Mammalian CELF/Bruno-like RNA-binding proteins: molecular characteristics and biological functions. Biochimie. 2006;88:515–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

