Dopamine Modulates Motor Control in a Specific Plane Related to Support
- PMID: 27145032
- PMCID: PMC4856377
- DOI: 10.1371/journal.pone.0155058
Dopamine Modulates Motor Control in a Specific Plane Related to Support
Abstract
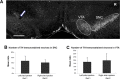
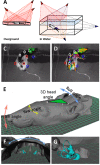
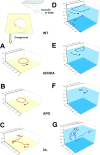
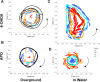
At the acute stage following unilateral labyrinthectomy (UL), rats, mice or guinea pigs exhibit a complex motor syndrome combining circling (HSCC lesion) and rolling (utricular lesion). At the chronic stage, they only display circling, because proprioceptive information related to the plane of support substitutes the missing utricular information to control posture in the frontal plane. Circling is also observed following unilateral lesion of the mesencephalic dopaminergic neurons by 6- hydroxydopamine hydrobromide (6-OHDA rats) and systemic injection of apomorphine (APO rats). The resemblance of behavior induced by unilateral vestibular and dopaminergic lesions at the chronic stage can be interpreted in two ways. One hypothesis is that the dopaminergic system exerts three-dimensional control over motricity, as the vestibular system does. If this hypothesis is correct, then a unilateral lesion of the nigro-striatal pathway should induce three-dimensional motor deficits, i.e., circling and at least some sort of barrel rolling at the acute stage of the lesion. Then, compensation could also take place very rapidly based on proprioception, which would explain the prevalence of circling. In addition, barrel rolling should reappear when the rodent is placed in water, as it occurs in UL vertebrates. Alternatively, the dopaminergic network, together with neurons processing the horizontal canal information, could control the homeostasis of posture and locomotion specifically in one and only one plane of space, i.e. the plane related to the basis of support. In that case, barrel rolling should never occur, whether at the acute or chronic stage on firm ground or in water. Moreover, circling should have the same characteristics following both types of lesions. Clearly, 6-OHDA and APO-rats never exhibited barrel rolling at the acute stage. They circled at the acute stage of the lesion and continued to do so three weeks later, including in water. In contrast, UL-rats, exhibited both circling and barrel rolling at the acute stage, and then only circled on the ground. Furthermore, barrel rolling instantaneously reappeared in water in UL rats, which was not the case in 6-OHDA and APO-rats. That is, the lesion of the dopaminergic system on one side did not compromise trim in the pitch and roll planes, even when proprioceptive information related to the basis of support was lacking as in water. Altogether, these results strongly suggest that dopamine does not exert three-dimensional control of the motor system but regulates postural control in one particular plane of space, the one related to the basis of support. In contrast, as previously shown, the vestibular system exerts three-dimensional control on posture. That is, we show here for the first time a relationship between a given neuromodulator and the spatial organization of motor control.
Conflict of interest statement
Figures
References
-
- de Waele C, Graf W, Josset P, Vidal PP. A radiological analysis of the postural syndromes following hemilabyrinthectomy and selective canal and otolith lesions in the guinea pig. Exp Brain Res. 1989; 77(1):166–182. - PubMed
-
- Vidal P-P, Wang DH, Graf W, de Waele C. Vestibular control of skeletal geometry in the guinea pig: a probleme of good trim? Prog Brain Res. 1993; 97:229–243. - PubMed
-
- Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971; 367:69–93. - PubMed
-
- Glick SD, Jerusssi TP, Fleisher LN. Turning in cycle: The neuropharmacology of rotation. Life Sci. 1976; 18:889–896. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

