A novel de novo dominant negative mutation in DNM1L impairs mitochondrial fission and presents as childhood epileptic encephalopathy
- PMID: 27145208
- PMCID: PMC5100740
- DOI: 10.1002/ajmg.a.37721
A novel de novo dominant negative mutation in DNM1L impairs mitochondrial fission and presents as childhood epileptic encephalopathy
Abstract
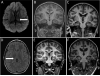
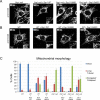
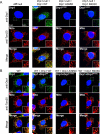
DNM1L encodes dynamin-related protein 1 (DRP1/DLP1), a key component of the mitochondrial fission machinery that is essential for proper functioning of the mammalian brain. Previously reported probands with de novo missense mutations in DNM1L presented in the first year of life with severe encephalopathy and refractory epilepsy, with several dying within the first several weeks after birth. In contrast, we report identical novel missense mutations in DNM1L in two unrelated probands who experienced normal development for several years before presenting with refractory focal status epilepticus and subsequent rapid neurological decline. We expand the phenotype of DNM1L-related mitochondrial fission defects, reveal common unique clinical characteristics and imaging findings, and compare the cellular impact of this novel mutation to the previously reported A395D lethal variant. We demonstrate that our R403C mutation, which resides in the assembly region of DRP1, acts by a dominant-negative mechanism and reduces oligomerization, mitochondrial fission activity, and mitochondrial recruitment of DRP1, but to a lesser extent compared to the A395D mutation. In contrast to the initial report of neonatal lethality resulting from DNM1L mutation and DRP1 dysfunction, our results show that milder DRP1 impairment is compatible with normal early development and subsequently results in a distinct set of neurological findings. In addition, we identify a common pathogenic mechanism whereby DNM1L mutations impair mitochondrial fission. © 2016 Wiley Periodicals, Inc.
Keywords: DLP1; DNM1L; DRP1; developmental regression; epileptic encephalopathy; fission; mitochondria; seizures.
© 2016 Wiley Periodicals, Inc.
Figures

References
-
- Bitoun M, Bevilacqua JA, Prudhon B, Maugenre S, Taratuto AL, Monges S, Lubieniecki F, Cances C, Uro-Coste E, Mayer M, Fardeau M, Romero NB, Guicheney P. Dynamin 2 mutations cause sporadic centronuclear myopathy with neonatal onset. Ann Neurol. 2007;62(6):666–670. - PubMed
-
- Chan DC. Mitochondrial dynamics in disease. N Engl J Med. 2007;356(17):1707–1709. - PubMed
-
- Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–287. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

