R- and S-citalopram concentrations have differential effects on neuropsychiatric scores in elders with dementia and agitation
- PMID: 27145364
- PMCID: PMC6445501
- DOI: 10.1111/bcp.12997
R- and S-citalopram concentrations have differential effects on neuropsychiatric scores in elders with dementia and agitation
Abstract
Aims: The aim was to determine the relationship between (R) and (S)-citalopram enantiomer exposure (AUC(0,24 h)) and therapeutic response in agitated individuals greater than 60 years old with Alzheimer's dementia (AD).
Methods: Citalopram enantiomer exposures (AUC(0,24 h)) derived from an established population pharmacokinetic analysis were utilized to explore the relationship between (R)- and (S)-citalopram area under the curve (AUC(0,24 )) and Mini-Mental State Examination (MMSE), Neurobehavioural Rating Scale-Agitation Subscale (NBRS-A), modified Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change (mADCS-CGIC) and Neuropsychiatric Inventory Agitation subscale (NPIA) scores. Time dependent changes in these scores (disease progression) were accounted for prior to exploring the exposure effect relationship for each enantiomer. These relationships were evaluated using a non-linear-mixed effects modelling approach as implemented in nonmem v7.3.
Results: (S)-AUC(0,24 h) and (R)-AUC(0,24 h) each contributed to improvement in NBRS-A scores (k3(R) -0.502; k4(S) -0.712) as did time in treatment. However, increasing (R)-AUC(0,24 h) decreased the probability of patient response (maximum Δ -0.182%/AUC(0,24 h)) based on the CGIC while (S)-AUC(0,24 h) improved the probability of response (maximum Δ 0.112%/AUC(0,24 h)). (R)-AUC(0,24 h) was also associated with worsening in MMSE scores (-0.5 points).
Conclusions: Our results suggest that citalopram enantiomers contributed differentially to treatment outcomes. (R)-citalopram accounted for a greater proportion of the adverse consequences associated with racemic citalopram treatment in patients with AD including a decreased probability of treatment response as measured by the CGIC and a reduction in MMSE scores. The S-enantiomer was associated with increased probability of response based on the CGIC.
Keywords: Alzheimer's disease; agitation; citalopram; escitalopram; pharmacodynamics.
© 2016 The British Pharmacological Society.
Figures
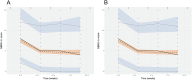
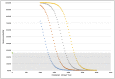
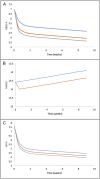
 placebo,
placebo,  (R)‐citalopram,
(R)‐citalopram,  (S)‐citalopram
(S)‐citalopramSimilar articles
-
Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial.JAMA. 2014 Feb 19;311(7):682-91. doi: 10.1001/jama.2014.93. JAMA. 2014. PMID: 24549548 Free PMC article. Clinical Trial.
-
Citalopram for the Treatment of Agitation in Alzheimer Dementia: Genetic Influences.J Geriatr Psychiatry Neurol. 2016 Mar;29(2):59-64. doi: 10.1177/0891988715601735. Epub 2015 Aug 23. J Geriatr Psychiatry Neurol. 2016. PMID: 26303700 Free PMC article. Clinical Trial.
-
Time to Response to Citalopram Treatment for Agitation in Alzheimer Disease.Am J Geriatr Psychiatry. 2015 Nov;23(11):1127-33. doi: 10.1016/j.jagp.2015.05.006. Epub 2015 May 19. Am J Geriatr Psychiatry. 2015. PMID: 26238225 Free PMC article. Clinical Trial.
-
When and How to Treat Agitation in Alzheimer's Disease Dementia With Citalopram and Escitalopram.Am J Geriatr Psychiatry. 2019 Oct;27(10):1099-1107. doi: 10.1016/j.jagp.2019.04.016. Epub 2019 May 10. Am J Geriatr Psychiatry. 2019. PMID: 31288974 Review.
-
Differences in the dynamics of serotonin reuptake transporter occupancy may explain superior clinical efficacy of escitalopram versus citalopram.Int Clin Psychopharmacol. 2009 May;24(3):119-25. doi: 10.1097/YIC.0b013e32832a8ec8. Int Clin Psychopharmacol. 2009. PMID: 19367152 Review.
Cited by
-
Dementia-related agitation: a review of non-pharmacological interventions and analysis of risks and benefits of pharmacotherapy.Transl Psychiatry. 2017 Oct 31;7(10):e1250. doi: 10.1038/tp.2017.199. Transl Psychiatry. 2017. PMID: 29087372 Free PMC article. Review.
-
Emerging Pharmacological Approaches for Psychosis and Agitation in Alzheimer's Disease.CNS Drugs. 2025 Feb;39(2):143-160. doi: 10.1007/s40263-024-01133-9. Epub 2024 Dec 2. CNS Drugs. 2025. PMID: 39623197 Free PMC article. Review.
-
Serotonin 2 Receptors, Agomelatine, and Behavioral and Psychological Symptoms of Dementia in Alzheimer's Disease.Behav Neurol. 2021 Mar 31;2021:5533827. doi: 10.1155/2021/5533827. eCollection 2021. Behav Neurol. 2021. PMID: 33859767 Free PMC article. Review.
-
Escitalopram Personalized Dosing: A Population Pharmacokinetics Repository Method.Drug Des Devel Ther. 2023 Sep 27;17:2955-2967. doi: 10.2147/DDDT.S425654. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37789969 Free PMC article. Review.
-
Management of Behavioral and Psychological Symptoms of Dementia.Curr Psychiatry Rep. 2019 Jul 1;21(8):66. doi: 10.1007/s11920-019-1049-5. Curr Psychiatry Rep. 2019. PMID: 31264056 Review.
References
-
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta‐analysis of randomized placebo‐controlled trials. JAMA 2005; 294: 1934–43. - PubMed
-
- Ballard C, Howard R. Neuroleptic drugs in dementia: benefits and harm. Nat Rev Neurosci 2006; 7: 492–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

