Wheat transcription factor TaWRKY70 is positively involved in high-temperature seedling plant resistance to Puccinia striiformis f. sp. tritici
- PMID: 27145738
- PMCID: PMC6638234
- DOI: 10.1111/mpp.12425
Wheat transcription factor TaWRKY70 is positively involved in high-temperature seedling plant resistance to Puccinia striiformis f. sp. tritici
Abstract
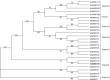
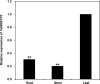
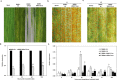
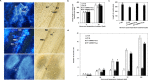
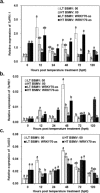
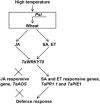
Stripe rust, caused by Puccinia striiformis f. sp. tritici (Pst), is a devastating disease of wheat (Triticum aestivum) worldwide. Wheat high-temperature seedling plant (HTSP) resistance to Pst is non-race-specific and durable. WRKY transcription factors have been proven to play important roles in plant defence responses to attacks by several pathogens. However, there is no direct evidence as to whether WRKY transcription factors play a role in HTSP resistance to Pst. We isolated a WRKY gene, named TaWRKY70, from wheat cultivar Xiaoyan 6. The expression level of TaWRKY70 was increased significantly when exposed to high temperatures (HTs) during the initial symptom expression stage of Pst infection. The expression of this gene increased in plants treated with ethylene (ET), salicylic acid (SA) and cold (4°C) stresses, but decreased in plants treated with methyl jasmonate (MeJA) and heat (40°C) stresses. Silencing of TaWRKY70 led to greater susceptibility to Pst (in terms of the increase in length of uredinial pustules and the decrease in the number of necrotic cells) compared with non-silenced plants when exposed to HT during the initial symptom expression stage of Pst infection, coinciding with expression changes of the ET- and SA-responsive genes TaPIE1 and TaPR1.1. In contrast, the expression level of the jasmonic acid (JA)-responsive gene TaAOS was not affected by TaWRKY70. These results indicate that TaWRKY70 is positively involved in HTSP resistance, during which SA and ET signalling are probably activated.
Keywords: Puccinia striiformis f. sp. tritici; WRKY70 transcription factor; high-temperature seedling plant resistance; virus-induced gene silencing.
© 2016 BSPP AND JOHN WILEY & SONS LTD.
Figures


Similar articles
-
The wheat WRKY transcription factors TaWRKY49 and TaWRKY62 confer differential high-temperature seedling-plant resistance to Puccinia striiformis f. sp. tritici.PLoS One. 2017 Jul 25;12(7):e0181963. doi: 10.1371/journal.pone.0181963. eCollection 2017. PLoS One. 2017. PMID: 28742872 Free PMC article.
-
The RLK protein TaCRK10 activates wheat high-temperature seedling-plant resistance to stripe rust through interacting with TaH2A.1.Plant J. 2021 Dec;108(5):1241-1255. doi: 10.1111/tpj.15513. Epub 2021 Oct 7. Plant J. 2021. PMID: 34583419
-
TaXa21, a Leucine-Rich Repeat Receptor-Like Kinase Gene Associated with TaWRKY76 and TaWRKY62, Plays Positive Roles in Wheat High-Temperature Seedling Plant Resistance to Puccinia striiformis f. sp. tritici.Mol Plant Microbe Interact. 2019 Nov;32(11):1526-1535. doi: 10.1094/MPMI-05-19-0137-R. Epub 2019 Sep 17. Mol Plant Microbe Interact. 2019. PMID: 31237476
-
Wheat stripe (yellow) rust caused by Puccinia striiformis f. sp. tritici.Mol Plant Pathol. 2014 Jun;15(5):433-46. doi: 10.1111/mpp.12116. Mol Plant Pathol. 2014. PMID: 24373199 Free PMC article. Review.
-
The Biological Roles of Puccinia striiformis f. sp. tritici Effectors during Infection of Wheat.Biomolecules. 2023 May 26;13(6):889. doi: 10.3390/biom13060889. Biomolecules. 2023. PMID: 37371469 Free PMC article. Review.
Cited by
-
Transcriptomic Analysis Reveal the Molecular Mechanisms of Wheat Higher-Temperature Seedling-Plant Resistance to Puccinia striiformis f. sp. tritici.Front Plant Sci. 2018 Feb 28;9:240. doi: 10.3389/fpls.2018.00240. eCollection 2018. Front Plant Sci. 2018. PMID: 29541084 Free PMC article.
-
A R2R3 MYB Transcription Factor, TaMYB391, Is Positively Involved in Wheat Resistance to Puccinia striiformis f. sp. tritici.Int J Mol Sci. 2022 Nov 15;23(22):14070. doi: 10.3390/ijms232214070. Int J Mol Sci. 2022. PMID: 36430549 Free PMC article.
-
Phytohormone crosstalk in the host-Verticillium interaction.Plant Signal Behav. 2020 Oct 2;15(10):1803567. doi: 10.1080/15592324.2020.1803567. Epub 2020 Aug 10. Plant Signal Behav. 2020. PMID: 32772774 Free PMC article.
-
Transcriptome analysis provides insights into the bases of salicylic acid-induced resistance to anthracnose in sorghum.Plant Mol Biol. 2022 Sep;110(1-2):69-80. doi: 10.1007/s11103-022-01286-5. Epub 2022 Jul 6. Plant Mol Biol. 2022. PMID: 35793006
-
WRKY Transcription Factor Response to High-Temperature Stress.Plants (Basel). 2021 Oct 18;10(10):2211. doi: 10.3390/plants10102211. Plants (Basel). 2021. PMID: 34686020 Free PMC article. Review.
References
-
- Abbruscato, P. , Nepusz, T. , Mizzi, L. , Del Corvo, M. , Morandini, P. , Fumasoni, I. , Michel, C. , Paccanaro, A. , Guiderdoni, E. , Schaffrath, U. , Morel, J.B. , Piffanelli, P. and Faivre‐Rampant, O. (2012) OsWRKY22, a monocot WRKY gene, plays a role in the resistance response to blast. Mol. Plant Pathol. 13, 828–841. - PMC - PubMed
-
- AbuQamar, S. , Chen, X. , Dhawan, R. , Bluhm, B. , Salmeron, J. , Lam, S. , Dietrich, R.A. and Mengiste, T. (2006) Expression profiling and mutant analysis reveals complex regulatory networks involved in Arabidopsis response to Botrytis infection. Plant J. 48, 28–44. - PubMed
-
- Agrawal, G.K. , Rakwal, R. , Jwa, N.S. , Han, K.S. and Agrawal, V.P. (2002) Molecular cloning and mRNA expression analysis of the first rice jasmonate biosynthetic pathway gene allene oxide synthase. Plant Physiol. Biochem. 40, 771–782.
-
- Albrecht, C. , Boutrot, F. , Segonzac, C. , Schwessinger, B. , Gimenez‐Ibanez, S. , Chinchilla, D. , Rathjen, J.P. , De Vries, S.C. and Zipfel, C. (2012) Brassinosteroids inhibit pathogen‐associated molecular pattern‐triggered immune signaling independent of the receptor kinase BAK1. Proc . Natl. Acad. Sci. USA, 109, 303–308. - PMC - PubMed
-
- Alvarez‐Venegas, R. , Al Abdallat, A. , Guo, M. , Alfano, J.R. and Avramova, Z. (2007) Epigenetic control of a transcription factor at the cross section of two antagonistic pathways. Epigenetics, 2, 106–113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

