Regulation of the cerebral circulation: bedside assessment and clinical implications
- PMID: 27145751
- PMCID: PMC4857376
- DOI: 10.1186/s13054-016-1293-6
Regulation of the cerebral circulation: bedside assessment and clinical implications
Abstract
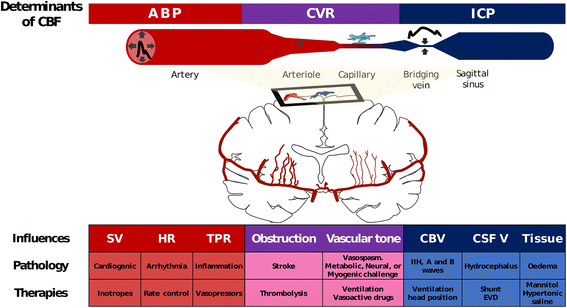
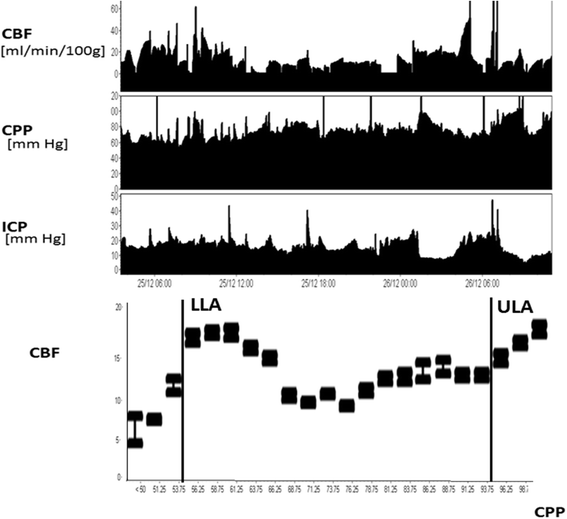
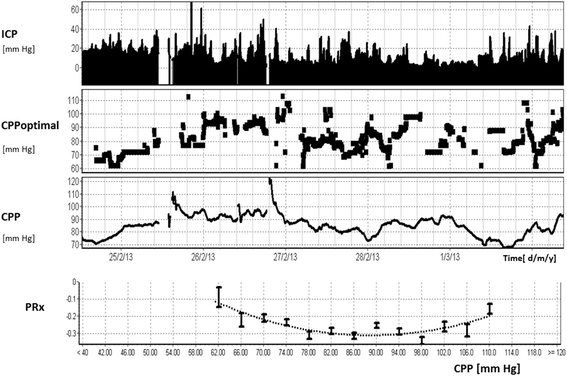
Regulation of the cerebral circulation relies on the complex interplay between cardiovascular, respiratory, and neural physiology. In health, these physiologic systems act to maintain an adequate cerebral blood flow (CBF) through modulation of hydrodynamic parameters; the resistance of cerebral vessels, and the arterial, intracranial, and venous pressures. In critical illness, however, one or more of these parameters can be compromised, raising the possibility of disturbed CBF regulation and its pathophysiologic sequelae. Rigorous assessment of the cerebral circulation requires not only measuring CBF and its hydrodynamic determinants but also assessing the stability of CBF in response to changes in arterial pressure (cerebral autoregulation), the reactivity of CBF to a vasodilator (carbon dioxide reactivity, for example), and the dynamic regulation of arterial pressure (baroreceptor sensitivity). Ideally, cerebral circulation monitors in critical care should be continuous, physically robust, allow for both regional and global CBF assessment, and be conducive to application at the bedside. Regulation of the cerebral circulation is impaired not only in primary neurologic conditions that affect the vasculature such as subarachnoid haemorrhage and stroke, but also in conditions that affect the regulation of intracranial pressure (such as traumatic brain injury and hydrocephalus) or arterial blood pressure (sepsis or cardiac dysfunction). Importantly, this impairment is often associated with poor patient outcome. At present, assessment of the cerebral circulation is primarily used as a research tool to elucidate pathophysiology or prognosis. However, when combined with other physiologic signals and online analytical techniques, cerebral circulation monitoring has the appealing potential to not only prognosticate patients, but also direct critical care management.
Figures




References
-
- Ursino M, Lodi CA. A simple mathematical model of the interaction between intracranial pressure and cerebral hemodynamics. J Appl Physiol. 1997;82:1256–69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

