Selective inhibition of tropomyosin-receptor-kinase A (TrkA) reduces pain and joint damage in two rat models of inflammatory arthritis
- PMID: 27145816
- PMCID: PMC4857260
- DOI: 10.1186/s13075-016-0996-z
Selective inhibition of tropomyosin-receptor-kinase A (TrkA) reduces pain and joint damage in two rat models of inflammatory arthritis
Abstract
Background: Inflammation is an essential component of arthritis pain. Nerve growth factor (NGF) plays a key role in acute and chronic pain states especially those associated with inflammation. NGF acts through tropomyosin-receptor-kinase A (TrkA). NGF blockade has reduced arthritis pain in clinical trials. We explored the mechanisms within the joint which may contribute to the analgesic effects of NGF by selectively inhibiting TrkA in carrageenan-induced or collagen-induced joint pain behaviour. The goal of the current study was to elucidate whether inflammation is central to the efficacy for NGF blockade.
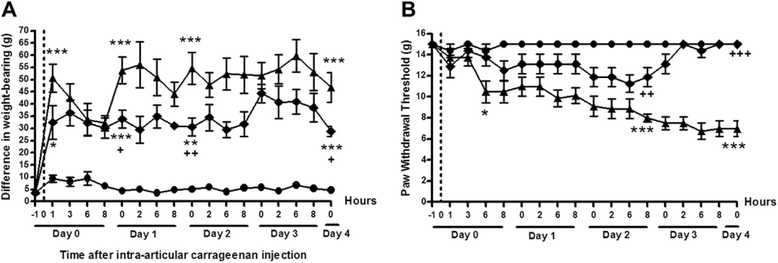
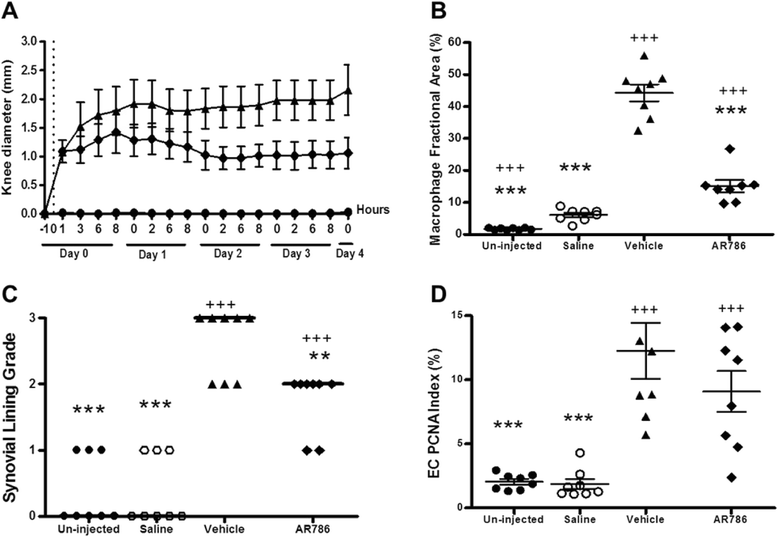
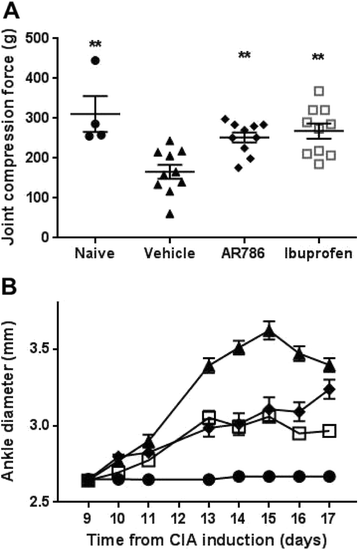
Methods: Rats were injected in their left knees with 2 % carrageenan or saline. Collagen-induced arthritis (CIA) was induced by intradermal injections of a mixture of bovine type II collagen (0.2 mg) and incomplete Freund's adjuvant (0.2 mg). Oral doses (30 mg/kg) of AR786 or vehicle control were given twice daily after arthritis induction. Ibuprofen-treated (35 mg/kg, orally, once daily) rats with CIA were used as positive analgesic controls. Pain behaviour was measured as hind-limb weight-bearing asymmetry and hind-paw withdrawal thresholds to von Frey hair stimulation (carrageenan synovitis), or withdrawal to joint compression using a Randall Selitto device (CIA). Inflammation was measured as increased knee joint diameter and by histopathological analysis.
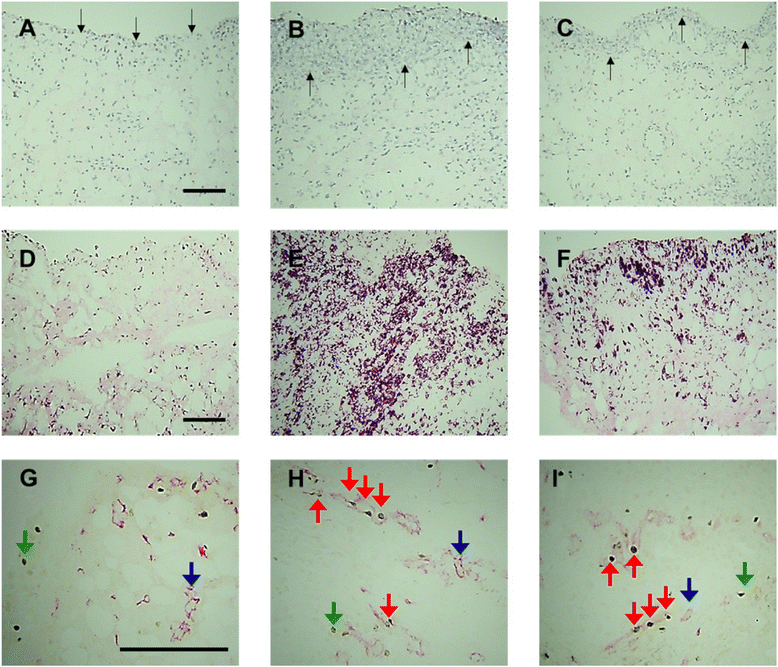
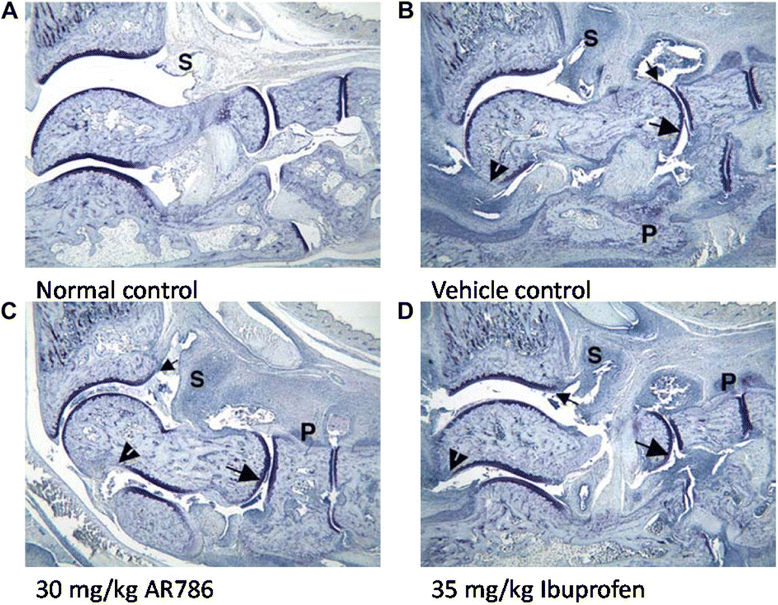
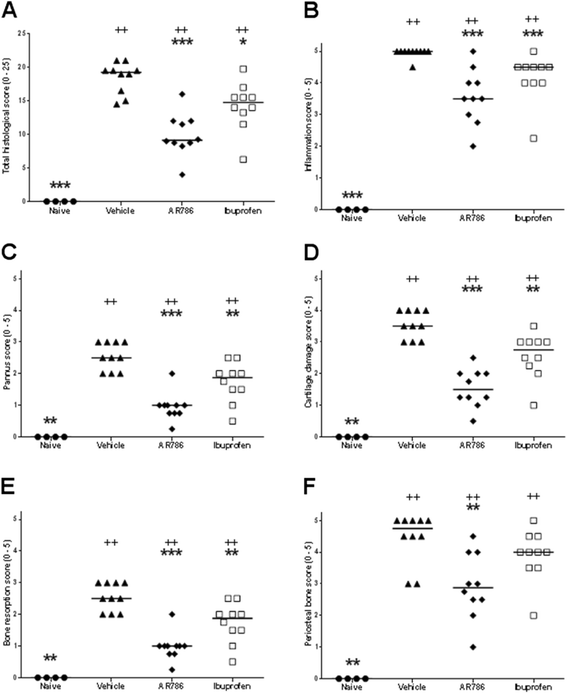
Results: Intra-articular injections of carrageenan or induction of CIA was each associated with pain behaviour and synovial inflammation. Systemic administration of the TrkA inhibitor AR786 reduced carrageenan-induced or CIA-induced pain behaviour to control values, and inhibited joint swelling and histological evidence of synovial inflammation and joint damage.
Conclusions: By using two models of varying inflammation we demonstrate for the first time that selective inhibition of TrkA may reduce carrageenan-induced or CIA-induced pain behaviour in rats, in part through potentially inhibiting synovial inflammation, although direct effects on sensory nerves are also likely. Our observations suggest that inflammatory arthritis causes pain and the presence of inflammation is fundamental to the beneficial effects (reduction in pain and pathology) of NGF blockade. Further research should determine whether TrkA inhibition may ameliorate human inflammatory arthritis.
Keywords: Carrageenan; Collagen-induced arthritis; Inflammation; Knee; Nerve growth factor; Pain; Tropomyosin-receptor-kinase A.
Figures
References
-
- Manni L, Lundeberg T, Fiorito S, Bonini S, Vigneti E, Aloe L. Nerve growth factor release by human synovial fibroblasts prior to and following exposure to tumor necrosis factor-alpha, interleukin-1 beta and cholecystokinin-8: the possible role of NGF in the inflammatory response. Clin Exp Rheumatol. 2003;21(5):617–24. - PubMed
-
- Ashraf S, Mapp PI, Walsh DA. Angiogenesis and the persistence of inflammation in a rat model of proliferative synovitis. Arthritis Rheum. 2010;62(7):1890–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

