The Impact of Experiencing Adverse Drug Reactions on the Patient's Quality of Life: A Retrospective Cross-Sectional Study in the Netherlands
- PMID: 27145946
- PMCID: PMC4933735
- DOI: 10.1007/s40264-016-0422-0
The Impact of Experiencing Adverse Drug Reactions on the Patient's Quality of Life: A Retrospective Cross-Sectional Study in the Netherlands
Abstract
Introduction: There is little information as to what extent adverse drug reactions (ADRs) influence patients' health-related quality of life (HR-QOL). From a pharmacovigilance perspective, capturing and making the best use of this information remains a challenge. The Netherlands Pharmacovigilance Centre Lareb received about 1800 reports after the packaging of the drug Thyrax(®) (levothyroxine; Aspen Pharma Trading Limited, Dublin, Ireland) changed from a brown glass bottle to a blister package in the Netherlands.
Objective: The objective of this study was to explore the impact of ADRs on HR-QOL in patients who reported a possible ADR to Lareb in relation to the change in the packaging of the drug Thyrax(®). A secondary objective was to explore factors correlated with change in HR-QOL.
Methods: Patients who reported an ADR in relation to the Thyrax(®) packaging change were included in this study. A web-based adapted version of the COOP/WONCA questionnaire was sent to explore the HR-QOL before versus during the ADR, expressed on a 5-point scale from no impact (1) to high impact (5). Multivariable linear regression analysis was used to identify factors correlated with change in HR-QOL.
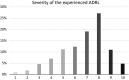
Results: Overall, 1167 patients returned the questionnaire (71.2 % response rate). The difference in HR-QOL was -0.8 for physical, -1.2 for mental, -1.4 for daily activities, -1.3 for social, and -1.3 for overall health status (p < 0.001 for each domain). Age, sex, educational level of the patient, and absence from work due to an ADR were correlated with at least one domain, while severity of the ADR was found to be correlated with all domains of HR-QOL.
Conclusion: Patients who reported possible ADRs after the Thyrax(®) packaging change experienced a significant decrease in HR-QOL. This impact was highest for the domains 'daily activities', 'overall health status', and 'mental health' and lowest for 'physical fitness'.
Figures
References
-
- Centers for Disease Control and Prevention. Measuring healthy days: population assessment of health-related quality of life. Atlanta: Centers for Disease Control and Prevention; 2000. http://www.cdc.gov/hrqol/pdfs/mhd.pdf. Accessed 21 June 2015.
-
- Reporting adverse drug reactions: definitions of terms and criteria for their use. Geneva: Counsel for International Organizations of Medical Sciences (CIOMS); 1999. http://www.cioms.ch/publications/reporting_adverse_drug.pdf. Accessed 1 Aug 2015.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials