Clinical Trial of an Oral Live Shigella sonnei Vaccine Candidate, WRSS1, in Thai Adults
- PMID: 27146000
- PMCID: PMC4933782
- DOI: 10.1128/CVI.00665-15
Clinical Trial of an Oral Live Shigella sonnei Vaccine Candidate, WRSS1, in Thai Adults
Abstract
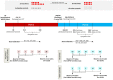
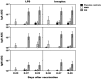
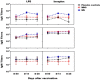
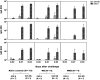
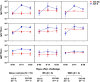
Live attenuated Shigella sonnei vaccine candidate WRSS1, previously tested in U.S. and Israeli volunteers, was evaluated in a population of adult Thai volunteers in which the organism is endemic. In a randomized placebo-controlled, double-blind design, inpatient participants received a single oral dose of 1.6 × 10(4) CFU of WRSS1. The vaccine was generally well tolerated, with equal numbers of vaccinees and placebo controls showing mild symptoms. Only 3 of 13 vaccinees (23%) had culture-positive stools, while a total of 9 vaccinees were positive by PCR. Lack of vaccine shedding in volunteers correlated with lack of clinical symptoms and immune responses, just as the duration of fecal shedding correlated directly with stronger immune responses. Two months following immunization, 10 vaccinees and 10 newly recruited naive controls received a challenge dose of 1,670 CFU of virulent S. sonnei strain 53G. This dose had previously demonstrated a 75% attack rate for dysentery in Thai volunteers. However, in this study the attack rate for dysentery in naive controls after challenge was 20%. Based on clinical record summaries, 3 vaccinees and 5 naive controls experienced clinically relevant illness (diarrhea/dysentery/fever/shigellosis), and a 40% vaccine efficacy was calculated. When these data are compared to those for the performance of this vaccine candidate in more naive populations, it is clear that a single oral dose of WRSS1 at 10(4) CFU failed to achieve its full potential in a population in which the organism is endemic. Higher doses and/or repeated immunizations may contribute to improved vaccine shedding and consequent elevation of protective immune responses in a population in which the organism is endemic. (The study has been registered at ClinicalTrials.gov under registration no. NCT01080716.).
Copyright © 2016 Pitisuttithum et al.
Figures
References
-
- von Seidlein L, Kim DR, Ali M, Lee H, Wang X, Thiem VD, Canh do G, Chaicumpa W, Agtini MD, Hossain A, Bhutta ZA, Mason C, Sethabutr O, Talukder K, Nair GB, Deen JL, Kotloff K, Clemens J. 2006. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med 3:e353. doi:10.1371/journal.pmed.0030353. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

