Rupture Forces among Human Blood Platelets at different Degrees of Activation
- PMID: 27146004
- PMCID: PMC4857101
- DOI: 10.1038/srep25402
Rupture Forces among Human Blood Platelets at different Degrees of Activation
Abstract
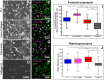
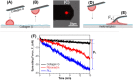
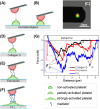
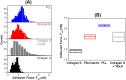
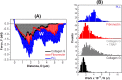
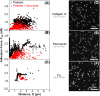
Little is known about mechanics underlying the interaction among platelets during activation and aggregation. Although the strength of a blood thrombus has likely major biological importance, no previous study has measured directly the adhesion forces of single platelet-platelet interaction at different activation states. Here, we filled this void first, by minimizing surface mediated platelet-activation and second, by generating a strong adhesion force between a single platelet and an AFM cantilever, preventing early platelet detachment. We applied our setup to measure rupture forces between two platelets using different platelet activation states, and blockade of platelet receptors. The rupture force was found to increase proportionally to the degree of platelet activation, but reduced with blockade of specific platelet receptors. Quantification of single platelet-platelet interaction provides major perspectives for testing and improving biocompatibility of new materials; quantifying the effect of drugs on platelet function; and assessing the mechanical characteristics of acquired/inherited platelet defects.
Figures
Similar articles
-
The key events of thrombus formation: platelet adhesion and aggregation.Biomech Model Mechanobiol. 2020 Jun;19(3):943-955. doi: 10.1007/s10237-019-01262-x. Epub 2019 Nov 21. Biomech Model Mechanobiol. 2020. PMID: 31754949
-
Activation induced morphological changes and integrin αIIbβ3 activity of living platelets.Methods. 2013 Apr 1;60(2):179-85. doi: 10.1016/j.ymeth.2013.03.034. Epub 2013 Apr 6. Methods. 2013. PMID: 23571313 Free PMC article.
-
Intermolecular force mapping of platelet surfaces on collagen substrata.J Biomed Mater Res. 1999 Jun 5;45(3):167-74. doi: 10.1002/(sici)1097-4636(19990605)45:3<167::aid-jbm2>3.0.co;2-7. J Biomed Mater Res. 1999. PMID: 10397972
-
[Platelet physiology. Advances in platelet reactivity. Review].Invest Clin. 1994 Mar;35(1):41-62. Invest Clin. 1994. PMID: 8054381 Review. Spanish.
-
Platelets in atherosclerosis and thrombosis.Handb Exp Pharmacol. 2012;(210):111-33. doi: 10.1007/978-3-642-29423-5_5. Handb Exp Pharmacol. 2012. PMID: 22918729 Review.
Cited by
-
Controlling Surface-Induced Platelet Activation by Agarose and Gelatin-Based Hydrogel Films.ACS Omega. 2021 Apr 13;6(16):10963-10974. doi: 10.1021/acsomega.1c00764. eCollection 2021 Apr 27. ACS Omega. 2021. PMID: 34056249 Free PMC article.
-
Mitoquinone (MitoQ) Inhibits Platelet Activation Steps by Reducing ROS Levels.Int J Mol Sci. 2020 Aug 27;21(17):6192. doi: 10.3390/ijms21176192. Int J Mol Sci. 2020. PMID: 32867213 Free PMC article.
-
Quantifying single-platelet biomechanics: An outsider's guide to biophysical methods and recent advances.Res Pract Thromb Haemost. 2020 Feb 17;4(3):386-401. doi: 10.1002/rth2.12313. eCollection 2020 Mar. Res Pract Thromb Haemost. 2020. PMID: 32211573 Free PMC article. Review.
-
Platelets as autonomous drones for hemostatic and immune surveillance.J Exp Med. 2017 Aug 7;214(8):2193-2204. doi: 10.1084/jem.20170879. J Exp Med. 2017. PMID: 28720569 Free PMC article. Review.
-
Statistical analysis of 3D localisation microscopy images for quantification of membrane protein distributions in a platelet clot model.PLoS Comput Biol. 2020 Jun 30;16(6):e1007902. doi: 10.1371/journal.pcbi.1007902. eCollection 2020 Jun. PLoS Comput Biol. 2020. PMID: 32603371 Free PMC article.
References
-
- Michelson A. D. Platelets. Academic Press/Elsevier (2013).
-
- Stadelmann W. K., Digenis A. G. & Tobin G. R. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg 176, 26S–38S (1998). - PubMed
-
- Nieswandt B., Pleines I. & Bender M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J Thromb Haemost 9, 92–104 (2011). - PubMed
-
- Nurden A. T., Nurden P., Sanchez M., Andia I. & Anitua E. Platelets and wound healing. Front Biosci 13, 3532–3548 (2008). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

