Metabolic Reprogramming and Dependencies Associated with Epithelial Cancer Stem Cells Independent of the Epithelial-Mesenchymal Transition Program
- PMID: 27146024
- PMCID: PMC4860823
- DOI: 10.1002/stem.2286
Metabolic Reprogramming and Dependencies Associated with Epithelial Cancer Stem Cells Independent of the Epithelial-Mesenchymal Transition Program
Abstract
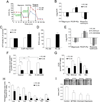
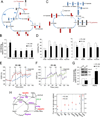
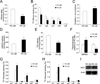
In solid tumors, cancer stem cells (CSCs) can arise independently of epithelial-mesenchymal transition (EMT). In spite of recent efforts, the metabolic reprogramming associated with CSC phenotypes uncoupled from EMT is poorly understood. Here, by using metabolomic and fluxomic approaches, we identify major metabolic profiles that differentiate metastatic prostate epithelial CSCs (e-CSCs) from non-CSCs expressing a stable EMT. We have found that the e-CSC program in our cellular model is characterized by a high plasticity in energy substrate metabolism, including an enhanced Warburg effect, a greater carbon and energy source flexibility driven by fatty acids and amino acid metabolism and an essential reliance on the proton buffering capacity conferred by glutamine metabolism. An analysis of transcriptomic data yielded a metabolic gene signature for our e-CSCs consistent with the metabolomics and fluxomics analyses that correlated with tumor progression and metastasis in prostate cancer and in 11 additional cancer types. Interestingly, an integrated metabolomics, fluxomics, and transcriptomics analysis allowed us to identify key metabolic players regulated at the post-transcriptional level, suggesting potential biomarkers and therapeutic targets to effectively forestall metastasis. Stem Cells 2016;34:1163-1176.
Keywords: Cancer stem cells; Epithelial-mesenchymal transition; Glutaminolysis; Metabolic flux analysis; Mitochondrial metabolism; Warburg effect.
© 2016 AlphaMed Press.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

