A Novel Protective Function of 5-Methoxytryptophan in Vascular Injury
- PMID: 27146795
- PMCID: PMC4857180
- DOI: 10.1038/srep25374
A Novel Protective Function of 5-Methoxytryptophan in Vascular Injury
Abstract
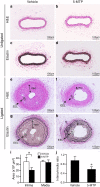
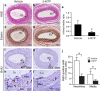
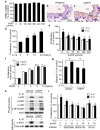
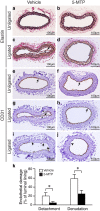
5-Methoxytryptophan (5-MTP), a 5-methoxyindole metabolite of tryptophan metabolism, was recently shown to suppress inflammatory mediator-induced cancer cell proliferation and migration. However, the role of 5-MTP in vascular disease is unknown. In this study, we investigated whether 5-MTP protects against vascular remodeling following arterial injury. Measurements of serum 5-MTP levels in healthy subjects and patients with coronary artery disease (CAD) showed that serum 5-MTP concentrations were inversely correlated with CAD. To test the role of 5-MTP in occlusive vascular disease, we subjected mice to a carotid artery ligation model of neointima formation and treated mice with vehicle or 5-MTP. Compared with vehicle-treated mice, 5-MTP significantly reduced intimal thickening by 40% 4 weeks after ligation. BrdU incorporation assays revealed that 5-MTP significantly reduced VSMC proliferation both in vivo and in vitro. Furthermore, 5-MTP reduced endothelial loss and detachment, ICAM-1 and VCAM-1 expressions, and inflammatory cell infiltration in the ligated arterial wall, suggesting attenuation of endothelial dysfunction. Signaling pathway analysis indicated that 5-MTP mediated its effects predominantly via suppressing p38 MAPK signaling in endothelial and VSMCs. Our data demonstrate a novel vascular protective function of 5-MTP against arterial injury-induced intimal hyperplasia. 5-MTP might be a therapeutic target for preventing and/or treating vascular remodeling.
Figures

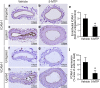

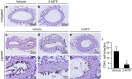
References
-
- Owens G. K., Kumar M. S. & Wamhoff B. R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 84, 767–801 (2004). - PubMed
-
- Ross R. & Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science 180, 1332–1339 (1973). - PubMed
-
- Donners M. M., Daemen M. J., Cleutjens K. B. & Heeneman S. Inflammation and restenosis: implications for therapy. Ann. Med. 35, 523–531 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

