Knockout of the BK β4-subunit promotes a functional coupling of BK channels and ryanodine receptors that mediate a fAHP-induced increase in excitability
- PMID: 27146987
- PMCID: PMC4978790
- DOI: 10.1152/jn.00857.2015
Knockout of the BK β4-subunit promotes a functional coupling of BK channels and ryanodine receptors that mediate a fAHP-induced increase in excitability
Abstract
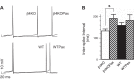
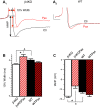
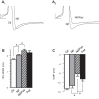
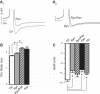
BK channels are large-conductance calcium- and voltage-activated potassium channels with diverse properties. Knockout of the accessory BK β4-subunit in hippocampus dentate gyrus granule neurons causes BK channels to change properties from slow-gated type II channels to fast-gated type I channels that sharpen the action potential, increase the fast afterhyperpolarization (fAHP) amplitude, and increase spike frequency. Here we studied the calcium channels that contribute to fast-gated BK channel activation and increased excitability of β4 knockout neurons. By using pharmacological blockers during current-clamp recording, we find that BK channel activation during the fAHP is dependent on ryanodine receptor activation. In contrast, L-type calcium channel blocker (nifedipine) affects the BK channel-dependent repolarization phase of the action potential but has no effect on the fAHP. Reducing BK channel activation during the repolarization phase with nifedipine, or during the fAHP with ryanodine, indicated that it is the BK-mediated increase of the fAHP that confers proexcitatory effects. The proexcitatory role of the fAHP was corroborated using dynamic current clamp. Increase or decrease of the fAHP amplitude during spiking revealed an inverse relationship between fAHP amplitude and interspike interval. Finally, we show that the seizure-prone ryanodine receptor gain-of-function (R2474S) knockin mice have an unaltered repolarization phase but larger fAHP and increased AP frequency compared with their control littermates. In summary, these results indicate that an important role of the β4-subunit is to reduce ryanodine receptor-BK channel functional coupling during the fAHP component of the action potential, thereby decreasing excitability of dentate gyrus neurons.
Keywords: action potentials; dentate gyrus; fast afterhyperpolarization; large-conductance calcium- and voltage-activated potassium channels.
Copyright © 2016 the American Physiological Society.
Figures
Similar articles
-
Modulation by the BK accessory β4 subunit of phosphorylation-dependent changes in excitability of dentate gyrus granule neurons.Eur J Neurosci. 2011 Sep;34(5):695-704. doi: 10.1111/j.1460-9568.2011.07799.x. Epub 2011 Aug 16. Eur J Neurosci. 2011. PMID: 21848922 Free PMC article.
-
A computational model for how the fast afterhyperpolarization paradoxically increases gain in regularly firing neurons.J Neurophysiol. 2018 Apr 1;119(4):1506-1520. doi: 10.1152/jn.00385.2017. Epub 2018 Jan 10. J Neurophysiol. 2018. PMID: 29357445
-
Downregulation of KCNMB4 expression and changes in BK channel subtype in hippocampal granule neurons following seizure activity.PLoS One. 2017 Nov 16;12(11):e0188064. doi: 10.1371/journal.pone.0188064. eCollection 2017. PLoS One. 2017. PMID: 29145442 Free PMC article.
-
Current understanding of iberiotoxin-resistant BK channels in the nervous system.Front Physiol. 2014 Oct 9;5:382. doi: 10.3389/fphys.2014.00382. eCollection 2014. Front Physiol. 2014. PMID: 25346692 Free PMC article. Review.
-
Control of anterior pituitary cell excitability by calcium-activated potassium channels.Mol Cell Endocrinol. 2018 Mar 5;463:37-48. doi: 10.1016/j.mce.2017.06.003. Epub 2017 Jun 5. Mol Cell Endocrinol. 2018. PMID: 28596131 Review.
Cited by
-
KCNMA1-linked channelopathy.J Gen Physiol. 2019 Oct 7;151(10):1173-1189. doi: 10.1085/jgp.201912457. Epub 2019 Aug 19. J Gen Physiol. 2019. PMID: 31427379 Free PMC article. Review.
-
Structural and Functional Coupling of Calcium-Activated BK Channels and Calcium-Permeable Channels Within Nanodomain Signaling Complexes.Front Physiol. 2022 Jan 14;12:796540. doi: 10.3389/fphys.2021.796540. eCollection 2021. Front Physiol. 2022. PMID: 35095560 Free PMC article. Review.
-
A role for corticotropin-releasing factor signaling in the lateral habenula and its modulation by early-life stress.Sci Signal. 2018 Mar 6;11(520):eaan6480. doi: 10.1126/scisignal.aan6480. Sci Signal. 2018. PMID: 29511121 Free PMC article.
-
Ca2+- and Voltage-Activated K+ (BK) Channels in the Nervous System: One Gene, a Myriad of Physiological Functions.Int J Mol Sci. 2023 Feb 8;24(4):3407. doi: 10.3390/ijms24043407. Int J Mol Sci. 2023. PMID: 36834817 Free PMC article. Review.
-
BK channel properties correlate with neurobehavioral severity in three KCNMA1-linked channelopathy mouse models.Elife. 2022 Jul 12;11:e77953. doi: 10.7554/eLife.77953. Elife. 2022. PMID: 35819138 Free PMC article.
References
-
- Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science 314: 615–620, 2006. - PubMed
-
- Berridge MJ. Neuronal calcium signaling. Neuron 21: 13–26, 1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

