Non-equilibrium dynamics and floral trait interactions shape extant angiosperm diversity
- PMID: 27147092
- PMCID: PMC4874697
- DOI: 10.1098/rspb.2015.2304
Non-equilibrium dynamics and floral trait interactions shape extant angiosperm diversity
Abstract
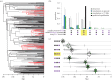
Why are some traits and trait combinations exceptionally common across the tree of life, whereas others are vanishingly rare? The distribution of trait diversity across a clade at any time depends on the ancestral state of the clade, the rate at which new phenotypes evolve, the differences in speciation and extinction rates across lineages, and whether an equilibrium has been reached. Here we examine the role of transition rates, differential diversification (speciation minus extinction) and non-equilibrium dynamics on the evolutionary history of angiosperms, a clade well known for the abundance of some trait combinations and the rarity of others. Our analysis reveals that three character states (corolla present, bilateral symmetry, reduced stamen number) act synergistically as a key innovation, doubling diversification rates for lineages in which this combination occurs. However, this combination is currently less common than predicted at equilibrium because the individual characters evolve infrequently. Simulations suggest that angiosperms will remain far from the equilibrium frequencies of character states well into the future. Such non-equilibrium dynamics may be common when major innovations evolve rarely, allowing lineages with ancestral forms to persist, and even outnumber those with diversification-enhancing states, for tens of millions of years.
Keywords: diversification; floral evolution; flower symmetry; macroevolution; non-equilibrium; pollination.
© 2016 The Author(s).
Figures
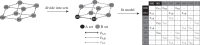
References
-
- Ree RH, Donoghue MJ. 1999. Inferring rates of change in flower symmetry in asterid angiosperms. Syst. Biol. 48, 633–641. (10.1080/106351599260201) - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

