Organic membranes determine the pattern of the columnar prismatic layer of mollusc shells
- PMID: 27147096
- PMCID: PMC4874705
- DOI: 10.1098/rspb.2016.0032
Organic membranes determine the pattern of the columnar prismatic layer of mollusc shells
Abstract
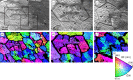
The degree to which biological control is exercised compared to physical control of the organization of biogenic materials is a central theme in biomineralization. We show that the outlines of biogenic calcite domains with organic membranes are always of simple geometries, while without they are much more complex. Moreover, the mineral prisms enclosed within the organic membranes are frequently polycrystalline. In the prismatic layer of the mollusc shell, organic membranes display a dynamics in accordance with the von Neumann-Mullins and Lewis Laws for two-dimensional foam, emulsion and grain growth. Taken together with the facts that we found instances in which the crystals do not obey such laws, and that the same organic membrane pattern can be found even without the mineral infilling, our work indicates that it is the membranes, not the mineral prisms, that control the pattern, and the mineral enclosed within the organic membranes passively adjusts to the dynamics dictated by the latter.
Keywords: biomineralization; mollusc; prismatic layer.
© 2016 The Author(s).
Figures




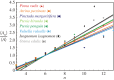
 in the CCP layer of bivalves where n is the number of sides of a prism,
in the CCP layer of bivalves where n is the number of sides of a prism,  is the average area of prisms of n sides,
is the average area of prisms of n sides,  is the average area of prisms. The species analysed belong to different orders and superfamilies of the bivalve subclass Pteriomorphia: order Pteroida, superfamily Pinnoidea (Pinna rudis (gradient α = 0.32), Atrina pectinata (α = 0.29)), superfamily Pterioidea (Pinctada margaritifera (α = 0.27), Pteria hirundo (α= 0.33), Pteria penguin (α = 0.34), Vulsella vulsella (α = 0.26), Isognomon isognomon (α = 0.39)), order Ostreoida, superfamily Ostreoidea (Ostrea edulis (α = 0.38)). The relationship is linear for the common prisms with good statistics whose number of sides is close to six.
is the average area of prisms. The species analysed belong to different orders and superfamilies of the bivalve subclass Pteriomorphia: order Pteroida, superfamily Pinnoidea (Pinna rudis (gradient α = 0.32), Atrina pectinata (α = 0.29)), superfamily Pterioidea (Pinctada margaritifera (α = 0.27), Pteria hirundo (α= 0.33), Pteria penguin (α = 0.34), Vulsella vulsella (α = 0.26), Isognomon isognomon (α = 0.39)), order Ostreoida, superfamily Ostreoidea (Ostrea edulis (α = 0.38)). The relationship is linear for the common prisms with good statistics whose number of sides is close to six.References
-
- Belcher AM, Wu XH, Christensen RJ, Hansma PK, Stucky GD, Morse DE. 1996. Control of crystal phase switching and orientation by soluble mollusc-shell proteins. Nature 381, 56–58. (10.1038/381056a0) - DOI
-
- Falini G, Albeck S, Weiner S, Addadi L. 1996. Control of aragonite or calcite polymorphism by mollusk shell macromolecules. Science 271, 67–69. (10.1126/science.271.5245.67) - DOI
-
- Mann S. 2001. Biomineralization. Principles and concepts in bioinorganic materials chemistry. Oxford, UK: Oxford University Press.
-
- Weiner S, Dove PM. 2003. An overview of biomineralization processes and the problem of the vital effect. Rev. Mineral. Geochem. 54, 1–29. (10.2113/0540001) - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

