Starch phosphorylation: insights and perspectives
- PMID: 27147464
- PMCID: PMC11108486
- DOI: 10.1007/s00018-016-2248-4
Starch phosphorylation: insights and perspectives
Abstract
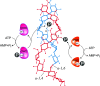
During starch metabolism, the phosphorylation of glucosyl residues of starch, to be more precise of amylopectin, is a repeatedly observed process. This phosphorylation is mediated by dikinases, the glucan, water dikinase (GWD) and the phosphoglucan, water dikinase (PWD). The starch-related dikinases utilize ATP as dual phosphate donor transferring the terminal γ-phosphate group to water and the β-phosphate group selectively to either C6 position or C3 position of a glucosyl residue within amylopectin. By the collaborative action of both enzymes, the initiation of a transition of α-glucans from highly ordered, water-insoluble state to a less order state is realized and thus the initial process of starch degradation. Consequently, mutants lacking either GWD or PWD reveal a starch excess phenotype as well as growth retardation. In this review, we focus on the increased knowledge collected over the last years related to enzymatic properties, the precise definition of the substrates, the physiological implications, and discuss ongoing questions.
Keywords: Glucan, water dikinase; Phosphoglucan, water dikinase; Starch degradation; Starch metabolism; Starch phosphorylation.
Figures


References
-
- Imberty A, Perez S. A revisit to the three-dimensional structure of b-type starch. Biopolymers. 1988;27(8):1205–1221. doi: 10.1002/bip.360270803. - DOI
-
- Deschamps P, Colleoni C, Nakamura Y, Suzuki E, Putaux JL, Buleon A, Haebel S, Ritte G, Steup M, Falcon LI, Moreira D, Loffelhardt W, Raj JN, Plancke C, d’Hulst C, Dauvillee D, Ball S. Metabolic symbiosis and the birth of the plant kingdom. Mol Biol Evol. 2008;25(3):536–548. doi: 10.1093/molbev/msm280. - DOI - PubMed
-
- Ball S. Evolution of the starch pathway. In: Tetlow IJ, editor. Starch: Origins, structure and metabolism. London: The Society for Experimental Biology; 2012. pp. 29–54.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

