The Hippo pathway in intestinal regeneration and disease
- PMID: 27147489
- PMCID: PMC5642988
- DOI: 10.1038/nrgastro.2016.59
The Hippo pathway in intestinal regeneration and disease
Abstract
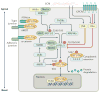
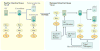
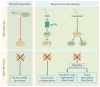
The Hippo pathway is a signalling cascade conserved from Drosophila melanogaster to mammals. The mammalian core kinase components comprise MST1 and MST2, SAV1, LATS1 and LATS2 and MOB1A and MOB1B. The transcriptional co-activators YAP1 and TAZ are the downstream effectors of the Hippo pathway and regulate target gene expression. Hippo signalling has crucial roles in the control of organ size, tissue homeostasis and regeneration, and dysregulation of the Hippo pathway can lead to uncontrolled cell growth and malignant transformation. Mammalian intestine consists of a stem cell compartment as well as differentiated cells, and its ability to regenerate rapidly after injury makes it an excellent model system to study tissue homeostasis, regeneration and tumorigenesis. Several studies have established the important role of the Hippo pathway in these processes. In addition, crosstalk between Hippo and other signalling pathways provides tight, yet versatile, regulation of tissue homeostasis. In this Review, we summarize studies on the role of the Hippo pathway in the intestine on these physiological processes and the underlying mechanisms responsible, and discuss future research directions and potential therapeutic strategies targeting Hippo signalling in intestinal disease.
Conflict of interest statement
The authors declare no competing interests.
Figures
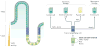
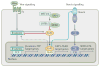
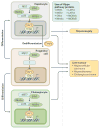
References
-
- Barry ER, Camargo FD. The Hippo superhighway: signaling crossroads converging on the Hippo/Yap pathway in stem cells and development. Curr Opin Cell Biol. 2013;25:247–253. - PubMed
-
- Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–1312. - PubMed
-
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. - PubMed
-
- Yu FX, Meng Z, Plouffe SW, Guan KL. Hippo pathway regulation of gastrointestinal tissues. Annu Rev Physiol. 2015;77:201–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

