Continuous anti-angiogenic therapy after tumor progression in patients with recurrent high-grade epithelial ovarian cancer: phase I trial experience
- PMID: 27147567
- PMCID: PMC5085215
- DOI: 10.18632/oncotarget.9048
Continuous anti-angiogenic therapy after tumor progression in patients with recurrent high-grade epithelial ovarian cancer: phase I trial experience
Abstract
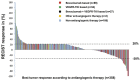
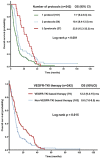
High-grade epithelial ovarian cancer (HG-EOC) is the most lethal gynecologic malignancy worldwide Once patients develop chemoresistance, effective novel strategies are required to improve prognosis We analyzed characteristics and outcomes of 242 consecutive patients with HG-EOC participating in 94 phase I clinical trials at The University of Texas MD Anderson Cancer Center. Baseline lactate dehydrogenase levels, albumin levels, and number of metastatic sites were independent predictors of overall survival (OS). Receiving more than 1 phase I protocol was associated with improved OS (p < 0.001). Regimens including a chemotherapeutic agent plus bevacizumab or Aurora A kinase inhibitor led to a median progression-free survival (PFS) duration of more than 6 months. Although patients receiving bevacizumab-based regimens in the phase I clinical trials had significantly longer PFS than those receiving other anti-angiogenic therapies (p = 0.017), patients treated with vascular endothelial growth factor receptor-tyrosine kinase inhibitors (VEGFR-TKIs) had significantly longer OS (12.2 months) than those not treated with VEGFR-TKIs (8.6 months, p = 0.015).In conclusion, anti-angiogenic therapy is one of the most important strategies for the treatment of HG-EOC, even in those who have already experienced tumor progression. Therefore, eligible patients with HG-EOC should be encouraged to participate in novel phase I studies of anti-angiogenic therapies, even after disease progression.
Keywords: anti-angiogenesis; epithelial ovarian cancer; overall survival; progression-free survival; tumor progression.
Conflict of interest statement
All authors have no potential conflicts of interest to disclose.
Figures

Similar articles
-
Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study.J Clin Oncol. 2007 Nov 20;25(33):5165-71. doi: 10.1200/JCO.2007.11.5345. J Clin Oncol. 2007. PMID: 18024863 Clinical Trial.
-
A phase II study of apatinib in patients with recurrent epithelial ovarian cancer.Gynecol Oncol. 2018 Feb;148(2):286-290. doi: 10.1016/j.ygyno.2017.12.013. Epub 2017 Dec 18. Gynecol Oncol. 2018. PMID: 29248198 Clinical Trial.
-
Phase II trial of nintedanib in patients with bevacizumab-resistant recurrent epithelial ovarian, tubal, and peritoneal cancer.Gynecol Oncol. 2019 Jun;153(3):555-561. doi: 10.1016/j.ygyno.2019.03.246. Epub 2019 Mar 28. Gynecol Oncol. 2019. PMID: 30929823 Clinical Trial.
-
An overview of tyrosine kinase inhibitors for the treatment of epithelial ovarian cancer.Expert Opin Investig Drugs. 2016;25(1):15-30. doi: 10.1517/13543784.2016.1117071. Epub 2015 Nov 26. Expert Opin Investig Drugs. 2016. PMID: 26560712 Review.
-
Efficacy of trebananib (AMG 386) in treating epithelial ovarian cancer.Expert Opin Pharmacother. 2016;17(6):853-60. doi: 10.1517/14656566.2016.1161027. Epub 2016 Mar 21. Expert Opin Pharmacother. 2016. PMID: 26933765 Review.
Cited by
-
Antiangiogenesis and gene aberration-related therapy may improve overall survival in patients with concurrent KRAS and TP53 hotspot mutant cancer.Oncotarget. 2017 May 16;8(20):33796-33806. doi: 10.18632/oncotarget.16840. Oncotarget. 2017. PMID: 28430579 Free PMC article.
-
MicroRNA-27b functions as a new inhibitor of ovarian cancer-mediated vasculogenic mimicry through suppression of VE-cadherin expression.RNA. 2017 Jul;23(7):1019-1027. doi: 10.1261/rna.059592.116. Epub 2017 Apr 10. RNA. 2017. PMID: 28396577 Free PMC article.
-
Aurora kinases in ovarian cancer.ESMO Open. 2020 Oct;5(5):e000718. doi: 10.1136/esmoopen-2020-000718. ESMO Open. 2020. PMID: 33087400 Free PMC article. Review.
-
Wise Management of Ovarian Cancer: On the Cutting Edge.J Pers Med. 2020 May 21;10(2):41. doi: 10.3390/jpm10020041. J Pers Med. 2020. PMID: 32455595 Free PMC article. Review.
-
Outcome analysis of Phase I trial patients with metastatic KRAS and/or TP53 mutant non-small cell lung cancer.Oncotarget. 2018 Sep 7;9(70):33258-33270. doi: 10.18632/oncotarget.25947. eCollection 2018 Sep 7. Oncotarget. 2018. PMID: 30279957 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. - PubMed
-
- Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. International journal of gynecological pathology. 2004;23:41–44. - PubMed
-
- Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, Verheijen RH, van der Burg ME, Lacave AJ, Panici PB, Kenter GG, Casado A, Mendiola C, Coens C, Verleye L, Stuart GC, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

