PKG II reverses HGF-triggered cellular activities by phosphorylating serine 985 of c-Met in gastric cancer cells
- PMID: 27147579
- PMCID: PMC5085148
- DOI: 10.18632/oncotarget.9074
PKG II reverses HGF-triggered cellular activities by phosphorylating serine 985 of c-Met in gastric cancer cells
Abstract
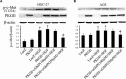
Previous studies showed that type II cGMP-dependent protein kinase G (PKG II) could inhibit the activation of epidermal growth factor receptor (EGFR). Both c-Met and EGFR belong to family of receptor tyrosine kinases (RTKs) and have high molecular analogy. However, the effect of PKG II on c-Met activation is unclear. This study was designed to investigate the inhibitory effect of PKG II on the activation of c-Met and consequent biological activities. The results from CCK8 assay, Transwell assay and TUNEL assay showed that HGF enhanced cell proliferation and migration, and decreased cell apoptosis. Activated PKG II reversed the above changes caused by HGF. Immunoprecipitation and Western blotting results showed that PKG II could bind with c-Met and phosphorylate its Ser985, and thereby inhibited HGF-induced activation of c-Met and MAPK/ERK and PI3K/Akt/mTOR mediated signal transduction. When Ser985 of c-Met was mutated to Alanine for preventing phosphorylation of this site, the blocking effect of PKG II on c-Met activation was annulled. When Ser985 of c-Met was mutated to Aspartic acid for mimicking phosphorylation of this site, HGF-induced activation of c-Met was prevented. In conclusion, the results indicated that PKG II could block c-Met activation via phosphorylating Ser985 of this RTK.
Keywords: c-Met; gastric cancer cell; inhibition; phosphorylation; type II cGMP-dependent protein kinase G.
Conflict of interest statement
There is no conflicts of interest.
Figures

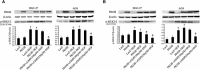

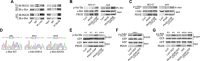
Similar articles
-
PKG II inhibits PDGF-BB triggered biological activities by phosphorylating PDGFRβ in gastric cancer cells.Cell Biol Int. 2018 Sep;42(10):1358-1369. doi: 10.1002/cbin.11020. Epub 2018 Jul 4. Cell Biol Int. 2018. PMID: 29935031
-
PKG II inhibits EGF/EGFR-induced migration of gastric cancer cells.PLoS One. 2013 Apr 16;8(4):e61674. doi: 10.1371/journal.pone.0061674. Print 2013. PLoS One. 2013. PMID: 23613900 Free PMC article.
-
Type II cGMP-dependent protein kinase directly inhibits HER2 activation of gastric cancer cells.Mol Med Rep. 2016 Feb;13(2):1909-13. doi: 10.3892/mmr.2015.4688. Epub 2015 Dec 17. Mol Med Rep. 2016. PMID: 26676300
-
Hepatocyte growth factor and Met in tumor biology and therapeutic approach with NK4.Proteomics. 2008 Aug;8(16):3360-70. doi: 10.1002/pmic.200800156. Proteomics. 2008. PMID: 18646008 Review.
-
Cellular and molecular mechanisms of HGF/Met in the cardiovascular system.Clin Sci (Lond). 2015 Dec;129(12):1173-93. doi: 10.1042/CS20150502. Clin Sci (Lond). 2015. PMID: 26561593 Review.
Cited by
-
Novel multiple tyrosine kinase inhibitor ponatinib inhibits bFGF-activated signaling in neuroblastoma cells and suppresses neuroblastoma growth in vivo.Oncotarget. 2017 Jan 24;8(4):5874-5884. doi: 10.18632/oncotarget.11580. Oncotarget. 2017. PMID: 27564113 Free PMC article.
-
CDKI-73: an orally bioavailable and highly efficacious CDK9 inhibitor against acute myeloid leukemia.Invest New Drugs. 2019 Aug;37(4):625-635. doi: 10.1007/s10637-018-0661-2. Epub 2018 Sep 8. Invest New Drugs. 2019. PMID: 30194564
-
Blockage of ETS homologous factor inhibits the proliferation and invasion of gastric cancer cells through the c-Met pathway.World J Gastroenterol. 2020 Dec 21;26(47):7497-7512. doi: 10.3748/wjg.v26.i47.7497. World J Gastroenterol. 2020. PMID: 33384550 Free PMC article.
-
Cyclic Guanosine Monophosphate (cGMP)-Dependent Protein Kinase II Blocks Epidermal Growth Factor (EGF)/Epidermal Growth Factor Receptor (EGFR)-Induced Biological Effects on Osteosarcoma Cells.Med Sci Monit. 2018 Apr 4;24:1997-2002. doi: 10.12659/msm.905892. Med Sci Monit. 2018. PMID: 29617357 Free PMC article.
-
Research progress on the development of hepatocyte growth factor/c-Met signaling pathway in gastric cancer: A review.World J Gastrointest Oncol. 2024 Aug 15;16(8):3397-3409. doi: 10.4251/wjgo.v16.i8.3397. World J Gastrointest Oncol. 2024. PMID: 39171189 Free PMC article. Review.
References
-
- Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330–1344. - PubMed
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Zhu C, Xu J, Li M, Zhao G, Cao H. Heterogeneity of c-Met expression in Chinese gastric cancer patients. Hum Pathol. 2015;46:1901–1907. - PubMed
-
- Marano L, Chiari R, Fabozzi A, De Vita F, Boccardi V, Roviello G, Petrioli R, Marrelli D, Roviello F, Patriti A. c-Met targeting in advanced gastric cancer: An open challenge. Cancer Lett. 2015;365:30–36. - PubMed
-
- Fuse N, Kuboki Y, Kuwata T, Nishina T, Kadowaki S, Shinozaki E, Machida N, Yuki S, Ooki A, Kajiura S, Kimura T, Yamanaka T, Shitara K, et al. Prognostic impact of HER2, EGFR, and c-MET status on overall survival of advanced gastric cancer patients. Gastric Cancer. 2016;19:183–191. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

