Synthesis of Self-Assembled Multifunctional Nanocomposite Catalysts with Highly Stabilized Reactivity and Magnetic Recyclability
- PMID: 27147586
- PMCID: PMC4857104
- DOI: 10.1038/srep25459
Synthesis of Self-Assembled Multifunctional Nanocomposite Catalysts with Highly Stabilized Reactivity and Magnetic Recyclability
Abstract
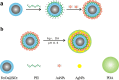
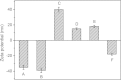
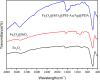
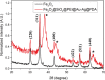
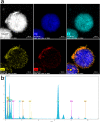
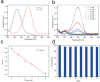
In this paper, a multifunctional Fe3O4@SiO2@PEI-Au/Ag@PDA nanocomposite catalyst with highly stabilized reactivity and magnetic recyclability was synthesized by a self-assembled method. The magnetic Fe3O4 nanoparticles were coated with a thin layer of the SiO2 to obtain a negatively charged surface. Then positively charged poly(ethyleneimine) polymer (PEI) was self-assembled onto the Fe3O4@SiO2 by electrostatic interaction. Next, negatively charged glutathione capped gold nanoparticles (GSH-AuNPs) were electrostatically self-assembled onto the Fe3O4@SiO2@PEI. After that, silver was grown on the surface of the nanocomposite due to the reduction of the dopamine in the alkaline solution. An about 5 nm thick layer of polydopamine (PDA) was observed to form the Fe3O4@SiO2@PEI-Au/Ag@PDA nanocomposite. The Fe3O4@SiO2@PEI-Au/Ag@PDA nanocomposite was carefully characterized by the SEM, TEM, FT-IR, XRD and so on. The Fe3O4@SiO2@PEI-Au/Ag@PDA nanocomposite shows a high saturation magnetization (Ms) of 48.9 emu/g, which allows it to be attracted rapidly to a magnet. The Fe3O4@SiO2@PEI-Au/Ag@PDA nanocomposite was used to catalyze the reduction of p-nitrophenol (4-NP) to p-aminophenol (4-AP) as a model system. The reaction kinetic constant k was measured to be about 0.56 min(-1) (R(2) = 0.974). Furthermore, the as-prepared catalyst can be easily recovered and reused for 8 times, which didn't show much decrease of the catalytic capability.
Figures



Similar articles
-
In situ loading of gold nanoparticles on Fe3O4@SiO2 magnetic nanocomposites and their high catalytic activity.Nanoscale. 2013 Jun 7;5(11):4894-901. doi: 10.1039/c3nr01075a. Epub 2013 Apr 26. Nanoscale. 2013. PMID: 23624783
-
Synthesis of Fe3O4@SiO2-Ag magnetic nanocomposite based on small-sized and highly dispersed silver nanoparticles for catalytic reduction of 4-nitrophenol.J Colloid Interface Sci. 2012 Oct 1;383(1):96-102. doi: 10.1016/j.jcis.2012.06.027. Epub 2012 Jun 19. J Colloid Interface Sci. 2012. PMID: 22789800
-
Synthesis and characterization of Fe3O4@SiO2@PDA@Ag core-shell nanoparticles and biological application on human lung cancer cell line and antibacterial strains.Artif Cells Nanomed Biotechnol. 2024 Dec;52(1):46-58. doi: 10.1080/21691401.2023.2295534. Epub 2023 Dec 29. Artif Cells Nanomed Biotechnol. 2024. PMID: 38156875
-
Silver nanoparticles decorated on thiol-modified magnetite nanoparticles (Fe3O4/SiO2-Pr-S-Ag) as a recyclable nanocatalyst for degradation of organic dyes.Mater Sci Eng C Mater Biol Appl. 2019 Apr;97:624-631. doi: 10.1016/j.msec.2018.12.076. Epub 2018 Dec 26. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30678949
-
Facile synthesis of Ag@Pd satellites-Fe3O4 core nanocomposites as efficient and reusable hydrogenation catalysts.Chem Commun (Camb). 2011 Nov 21;47(43):11924-6. doi: 10.1039/c1cc14675k. Epub 2011 Oct 5. Chem Commun (Camb). 2011. PMID: 21975908
Cited by
-
Magnetic domain interactions of Fe3O4 nanoparticles embedded in a SiO2 matrix.Sci Rep. 2018 Mar 23;8(1):5096. doi: 10.1038/s41598-018-23460-w. Sci Rep. 2018. PMID: 29572514 Free PMC article.
-
Photo-reduced Cu/CuO nanoclusters on TiO2 nanotube arrays as highly efficient and reusable catalyst.Sci Rep. 2017 Jan 10;7:39695. doi: 10.1038/srep39695. Sci Rep. 2017. PMID: 28071708 Free PMC article.
-
Synthesis Monitoring, Characterization and Cleanup of Ag-Polydopamine Nanoparticles Used as Antibacterial Agents with Field-Flow Fractionation.Antibiotics (Basel). 2022 Mar 8;11(3):358. doi: 10.3390/antibiotics11030358. Antibiotics (Basel). 2022. PMID: 35326821 Free PMC article.
-
Flavin Conjugated Polydopamine Nanoparticles Displaying Light-Driven Monooxygenase Activity.Front Chem. 2019 Apr 26;7:278. doi: 10.3389/fchem.2019.00278. eCollection 2019. Front Chem. 2019. PMID: 31080793 Free PMC article.
-
Facile Synthesis of Magnetically Recoverable Pd and Ru Catalysts for 4-Nitrophenol Reduction: Identifying Key Factors.ACS Omega. 2018 Nov 2;3(11):14717-14725. doi: 10.1021/acsomega.8b02382. eCollection 2018 Nov 30. ACS Omega. 2018. PMID: 31458148 Free PMC article.
References
-
- Wang D. S. & Li Y. D. One-pot protocol for Au-based hybrid magnetic nanostructures via a noble-metal-induced reduction process. J Am Chem Soc 132, 6280–6281 (2010). - PubMed
-
- Xu Z., Hou Y. & Sun S. Magnetic core/shell Fe3O4/Au and Fe3O4/Au/Ag nanoparticles with tunable plasmonic properties. J Am Chem Soc 129, 8698–8699 (2007). - PubMed
-
- Deng Y. et al. Multifunctional mesoporous composite microspheres with well-designed nanostructure: a highly integrated catalyst system. J Am Chem Soc 132, 8466–8473 (2010). - PubMed
-
- Liu R. et al. Dopamine as a carbon source: the controlled synthesis of hollow carbon spheres and yolk-structured carbon nanocomposites. Angew Chem Int Ed 50, 6799–6802 (2011). - PubMed
-
- Jiang H. L., Akita T., Ishida T., Haruta M. & Xu Q. Synergistic catalysis of Au@Ag core-shell nanoparticles stabilized on metal-organic framework. J Am Chem Soc 133, 1304–1306 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

