Pathophysiology of gadolinium-associated systemic fibrosis
- PMID: 27147669
- PMCID: PMC4967166
- DOI: 10.1152/ajprenal.00166.2016
Pathophysiology of gadolinium-associated systemic fibrosis
Abstract
Systemic fibrosis from gadolinium-based magnetic resonance imaging contrast is a scourge for the afflicted. Although gadolinium-associated systemic fibrosis is a rare condition, the threat of litigation has vastly altered clinical practice. Most theories concerning the etiology of the fibrosis are grounded in case reports rather than experiment. This has led to the widely accepted conjecture that the relative affinity of certain contrast agents for the gadolinium ion inversely correlates with the risk of succumbing to the disease. How gadolinium-containing contrast agents trigger widespread and site-specific systemic fibrosis and how chronicity is maintained are largely unknown. This review highlights experimentally-derived information from our laboratory and others that pertain to our understanding of the pathophysiology of gadolinium-associated systemic fibrosis.
Keywords: NADPH oxidase; fibrosis; gadolinium; nephrogenic fibrosing dermopathy; skin diseases.
Copyright © 2016 the American Physiological Society.
Figures

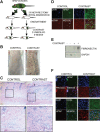

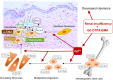
References
-
- Abujudeh HH, Kaewlai R, Kagan A, Chibnik LB, Nazarian RM, High WA, Kay J. Nephrogenic systemic fibrosis after gadopentetate dimeglumine exposure: case series of 36 patients. Radiology 253: 81–89, 2009. - PubMed
-
- Agarwal R, Brunelli SM, Williams K, Mitchell MD, Feldman HI, Umscheid CA. Gadolinium-based contrast agents and nephrogenic systemic fibrosis: a systematic review and meta-analysis. Nephrol Dial Transplant 24: 856–863, 2009. - PubMed
-
- Anavekar NS, Chong AH, Norris R, Dowling J, Goodman D. Nephrogenic systemic fibrosis in a gadolinium-naive renal transplant recipient. Australas J Dermatol 49: 44–47, 2008. - PubMed
-
- Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, Brenner DA. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56: 2316–2327, 2012. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

