Hepatitis C Virus-Induced Upregulation of MicroRNA miR-146a-5p in Hepatocytes Promotes Viral Infection and Deregulates Metabolic Pathways Associated with Liver Disease Pathogenesis
- PMID: 27147737
- PMCID: PMC4936128
- DOI: 10.1128/JVI.00619-16
Hepatitis C Virus-Induced Upregulation of MicroRNA miR-146a-5p in Hepatocytes Promotes Viral Infection and Deregulates Metabolic Pathways Associated with Liver Disease Pathogenesis
Abstract
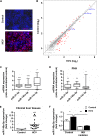
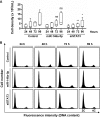
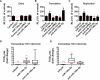
Hepatitis C virus (HCV)-induced chronic liver disease is a leading cause of hepatocellular carcinoma (HCC). However, the molecular mechanisms underlying HCC development following chronic HCV infection remain poorly understood. MicroRNAs (miRNAs) play an important role in homeostasis within the liver, and deregulation of miRNAs has been associated with liver disease, including HCC. While host miRNAs are essential for HCV replication, viral infection in turn appears to induce alterations of intrahepatic miRNA networks. Although the cross talk between HCV and liver cell miRNAs most likely contributes to liver disease pathogenesis, the functional involvement of miRNAs in HCV-driven hepatocyte injury and HCC remains elusive. Here we combined a hepatocyte-like cell-based model system, high-throughput small RNA sequencing, computational analysis, and functional studies to investigate HCV-miRNA interactions that may contribute to liver disease and HCC. Profiling analyses indicated that HCV infection differentially regulated the expression of 72 miRNAs by at least 2-fold, including miRNAs that were previously described to target genes associated with inflammation, fibrosis, and cancer development. Further investigation demonstrated that the miR-146a-5p level was consistently increased in HCV-infected hepatocyte-like cells and primary human hepatocytes, as well as in liver tissue from HCV-infected patients. Genome-wide microarray and computational analyses indicated that miR-146a-5p overexpression modulates pathways that are related to liver disease and HCC development. Furthermore, we showed that miR-146a-5p has a positive impact on late steps of the viral replication cycle, thereby increasing HCV infection. Collectively, our data indicate that the HCV-induced increase in miR-146a-5p expression both promotes viral infection and is relevant for pathogenesis of liver disease.
Importance: HCV is a leading cause of chronic liver disease and cancer. However, how HCV induces liver cancer remains poorly understood. There is accumulating evidence that a viral cure does not eliminate the risk for HCC development. Thus, there is an unmet medical need to develop novel approaches to predict and prevent virus-induced HCC. miRNA expression is known to be deregulated in liver disease and cancer. Furthermore, miRNAs are essential for HCV replication, and HCV infection alters miRNA expression. However, how miRNAs contribute to HCV-driven pathogenesis remains elusive. Here we show that HCV induces miRNAs that may contribute to liver injury and carcinogenesis. The miR-146a-5p level was consistently increased in different cell-based models of HCV infection and in HCV patient-derived liver tissue. Furthermore, miR-146a-5p increased HCV infection. Collectively, our data are relevant to understanding viral pathogenesis and may open perspectives for novel biomarkers and prevention of virus-induced liver disease and HCC.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, Heathcote EJ, Manns MP, Kuske L, Zeuzem S, Hofmann WP, de Knegt RJ, Hansen BE, Janssen HL. 2012. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 308:2584–2593. doi:10.1001/jama.2012.144878. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

