Weekly Paclitaxel Versus Three-Weekly Paclitaxel in Recurrent Platinum-Resistant Epithelial Ovarian and Peritoneal Cancers: A Phase III Study
- PMID: 27147900
- PMCID: PMC4847552
- DOI: 10.4137/CMO.S38204
Weekly Paclitaxel Versus Three-Weekly Paclitaxel in Recurrent Platinum-Resistant Epithelial Ovarian and Peritoneal Cancers: A Phase III Study
Abstract
Introduction: Treatment of recurrent platinum-resistant ovarian and peritoneal cancers represents a therapeutic challenge. The aim of this Phase III prospective study was to compare the survival benefits, objective response rate, and toxicities among patients treated by weekly paclitaxel with those who underwent three-weekly paclitaxel in recurrent platinum-resistant ovarian and peritoneal cancers.
Method: Patients with recurrent platinum-resistant ovarian and peritoneal cancer were allocated to receive either weekly paclitaxel (arm 1) at 80 m/m(2) or three-weekly paclitaxel (arm 2) at 175 mg/m(2).
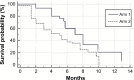
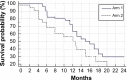
Results: Fifty-five patients were enrolled (30 arm 1, 25 arm 2). The mean age was 56.7 years, and the median performance status was 0 (Eastern Cooperative Oncology Group [ECOG]). For arms 1 and 2, the objective response rates were 27% and 16%, the median progression-free survival were 7 and 4.5 months, and the median overall survival were 15.5 and 12.5 months, respectively. Treatments also significantly improved the quality of life. Treatment was associated with mild toxicities, and while neuropathy was slightly higher for weekly paclitaxel over three-weekly paclitaxel, hematological toxicities were significantly lower for the former than the latter.
Conclusion: Paclitaxel rechallenge showed antitumor activity in recurrent platinum-resistant ovarian and peritoneal cancers. Weekly paclitaxel achieved better results than three-weekly paclitaxel in terms of survival benefits, quality of life, and toxicities.
Keywords: ORR; PFS; QOL; ovary; paclitaxel; platinum-resistant; weekly.
Figures
References
-
- Jemal A, Siegel R, Xu J, Ward E, Cancer WE. statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Pujade-Lauraine E, Paraiso D, Cure H, et al. Predicting the effectiveness of chemotherapy (Cx) in patients with recurrent ovarian cancer (ROC): a GINECO study. Proc Am Soc Clin Oncol. 2002:21. Abstract829.
-
- Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–8. - PubMed
-
- BCCA Protocol Summary. 2010. Available at: http://www.bccancer.bc.ca/HPI/Drug-Database/DrugIndexPro/Paclitaxel.htm.
LinkOut - more resources
Full Text Sources
Other Literature Sources