Inflammogenesis of Secondary Spinal Cord Injury
- PMID: 27147970
- PMCID: PMC4829593
- DOI: 10.3389/fncel.2016.00098
Inflammogenesis of Secondary Spinal Cord Injury
Abstract
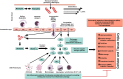
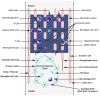
Spinal cord injury (SCI) and spinal infarction lead to neurological complications and eventually to paraplegia or quadriplegia. These extremely debilitating conditions are major contributors to morbidity. Our understanding of SCI has certainly increased during the last decade, but remains far from clear. SCI consists of two defined phases: the initial impact causes primary injury, which is followed by a prolonged secondary injury consisting of evolving sub-phases that may last for years. The underlying pathophysiological mechanisms driving this condition are complex. Derangement of the vasculature is a notable feature of the pathology of SCI. In particular, an important component of SCI is the ischemia-reperfusion injury (IRI) that leads to endothelial dysfunction and changes in vascular permeability. Indeed, together with endothelial cell damage and failure in homeostasis, ischemia reperfusion injury triggers full-blown inflammatory cascades arising from activation of residential innate immune cells (microglia and astrocytes) and infiltrating leukocytes (neutrophils and macrophages). These inflammatory cells release neurotoxins (proinflammatory cytokines and chemokines, free radicals, excitotoxic amino acids, nitric oxide (NO)), all of which partake in axonal and neuronal deficit. Therefore, our review considers the recent advances in SCI mechanisms, whereby it becomes clear that SCI is a heterogeneous condition. Hence, this leads towards evidence of a restorative approach based on monotherapy with multiple targets or combinatorial treatment. Moreover, from evaluation of the existing literature, it appears that there is an urgent requirement for multi-centered, randomized trials for a large patient population. These clinical studies would offer an opportunity in stratifying SCI patients at high risk and selecting appropriate, optimal therapeutic regimens for personalized medicine.
Keywords: glia; inflammation; ischemia-reperfusion injury (IRI); leukocytes; reactive oxygen species (ROS); spinal cord injury (SCI); therapeutics.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

