Modulation of Specific Sensory Cortical Areas by Segregated Basal Forebrain Cholinergic Neurons Demonstrated by Neuronal Tracing and Optogenetic Stimulation in Mice
- PMID: 27147975
- PMCID: PMC4837153
- DOI: 10.3389/fncir.2016.00028
Modulation of Specific Sensory Cortical Areas by Segregated Basal Forebrain Cholinergic Neurons Demonstrated by Neuronal Tracing and Optogenetic Stimulation in Mice
Abstract
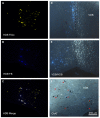
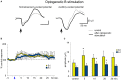
Neocortical cholinergic activity plays a fundamental role in sensory processing and cognitive functions. Previous results have suggested a refined anatomical and functional topographical organization of basal forebrain (BF) projections that may control cortical sensory processing in a specific manner. We have used retrograde anatomical procedures to demonstrate the existence of specific neuronal groups in the BF involved in the control of specific sensory cortices. Fluoro-Gold (FlGo) and Fast Blue (FB) fluorescent retrograde tracers were deposited into the primary somatosensory (S1) and primary auditory (A1) cortices in mice. Our results revealed that the BF is a heterogeneous area in which neurons projecting to different cortical areas are segregated into different neuronal groups. Most of the neurons located in the horizontal limb of the diagonal band of Broca (HDB) projected to the S1 cortex, indicating that this area is specialized in the sensory processing of tactile stimuli. However, the nucleus basalis magnocellularis (B) nucleus shows a similar number of cells projecting to the S1 as to the A1 cortices. In addition, we analyzed the cholinergic effects on the S1 and A1 cortical sensory responses by optogenetic stimulation of the BF neurons in urethane-anesthetized transgenic mice. We used transgenic mice expressing the light-activated cation channel, channelrhodopsin-2, tagged with a fluorescent protein (ChR2-YFP) under the control of the choline-acetyl transferase promoter (ChAT). Cortical evoked potentials were induced by whisker deflections or by auditory clicks. According to the anatomical results, optogenetic HDB stimulation induced more extensive facilitation of tactile evoked potentials in S1 than auditory evoked potentials in A1, while optogenetic stimulation of the B nucleus facilitated either tactile or auditory evoked potentials equally. Consequently, our results suggest that cholinergic projections to the cortex are organized into segregated pools of neurons that may modulate specific cortical areas.
Keywords: cholinergic facilitation; cholinergic projections; cortical evoked potentials; diagonal band of Broca; nucleus basalis magnocellularis; transgenic mice.
Figures






Similar articles
-
Basal Forebrain Nuclei Display Distinct Projecting Pathways and Functional Circuits to Sensory Primary and Prefrontal Cortices in the Rat.Front Neuroanat. 2018 Aug 15;12:69. doi: 10.3389/fnana.2018.00069. eCollection 2018. Front Neuroanat. 2018. PMID: 30158859 Free PMC article.
-
Bilateral Pathways from the Basal Forebrain to Sensory Cortices May Contribute to Synchronous Sensory Processing.Front Neuroanat. 2018 Jan 23;12:5. doi: 10.3389/fnana.2018.00005. eCollection 2018. Front Neuroanat. 2018. PMID: 29410616 Free PMC article.
-
Cholinergic inputs from Basal forebrain add an excitatory bias to odor coding in the olfactory bulb.J Neurosci. 2014 Mar 26;34(13):4654-64. doi: 10.1523/JNEUROSCI.5026-13.2014. J Neurosci. 2014. PMID: 24672011 Free PMC article.
-
Organization of the basal forebrain cholinergic system in the human brain.Handb Clin Neurol. 2025;211:11-21. doi: 10.1016/B978-0-443-19088-9.00009-3. Handb Clin Neurol. 2025. PMID: 40340056 Review.
-
Specific Basal Forebrain-Cortical Cholinergic Circuits Coordinate Cognitive Operations.J Neurosci. 2018 Oct 31;38(44):9446-9458. doi: 10.1523/JNEUROSCI.1676-18.2018. J Neurosci. 2018. PMID: 30381436 Free PMC article. Review.
Cited by
-
Role of Choline in Ocular Diseases.Int J Mol Sci. 2021 Apr 29;22(9):4733. doi: 10.3390/ijms22094733. Int J Mol Sci. 2021. PMID: 33946979 Free PMC article. Review.
-
Basal Forebrain Nuclei Display Distinct Projecting Pathways and Functional Circuits to Sensory Primary and Prefrontal Cortices in the Rat.Front Neuroanat. 2018 Aug 15;12:69. doi: 10.3389/fnana.2018.00069. eCollection 2018. Front Neuroanat. 2018. PMID: 30158859 Free PMC article.
-
A novel carbon tipped single micro-optrode for combined optogenetics and electrophysiology.PLoS One. 2018 Mar 7;13(3):e0193836. doi: 10.1371/journal.pone.0193836. eCollection 2018. PLoS One. 2018. PMID: 29513711 Free PMC article.
-
Input dependent modulation of olfactory bulb activity by HDB GABAergic projections.Sci Rep. 2020 Jul 1;10(1):10696. doi: 10.1038/s41598-020-67276-z. Sci Rep. 2020. PMID: 32612119 Free PMC article.
-
Cell-specific modulation of plasticity and cortical state by cholinergic inputs to the visual cortex.J Physiol Paris. 2016 Sep;110(1-2):37-43. doi: 10.1016/j.jphysparis.2016.11.004. Epub 2016 Nov 10. J Physiol Paris. 2016. PMID: 27840211 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

