New Molecular Insights of Insulin in Diabetic Cardiomyopathy
- PMID: 27148064
- PMCID: PMC4828458
- DOI: 10.3389/fphys.2016.00125
New Molecular Insights of Insulin in Diabetic Cardiomyopathy
Abstract
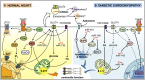
Type 2 diabetes mellitus (T2DM) is a highly prevalent disease worldwide. Cardiovascular disorders generated as a consequence of T2DM are a major cause of death related to this disease. Diabetic cardiomyopathy (DCM) is characterized by the morphological, functional and metabolic changes in the heart produced as a complication of T2DM. This cardiac disorder is characterized by constant high blood glucose and lipids levels which eventually generate oxidative stress, defective calcium handling, altered mitochondrial function, inflammation and fibrosis. In this context, insulin is of paramount importance for cardiac contractility, growth and metabolism and therefore, an impaired insulin signaling plays a critical role in the DCM development. However, the exact pathophysiological mechanisms leading to DCM are still a matter of study. Despite the numerous questions raised in the study of DCM, there have also been important findings, such as the role of micro-RNAs (miRNAs), which can not only have the potential of being important biomarkers, but also therapeutic targets. Furthermore, exosomes also arise as an interesting variable to consider, since they represent an important inter-cellular communication mechanism and therefore, they may explain many aspects of the pathophysiology of DCM and their study may lead to the development of therapeutic agents capable of improving insulin signaling. In addition, adenosine and adenosine receptors (ARs) may also play an important role in DCM. Moreover, the possible cross-talk between insulin and ARs may provide new strategies to reverse its defective signaling in the diabetic heart. This review focuses on DCM, the role of insulin in this pathology and the discussion of new molecular insights which may help to understand its underlying mechanisms and generate possible new therapeutic strategies.
Keywords: adenosine; adenosine receptors; diabetic cardiomyopathy; exosomes; insulin; miRNAs.
Figures
References
-
- Abel E. D., Graveleau C., Betuing S., Pham M., Reay P. A., Kandror V., et al. . (2004). Regulation of insulin-responsive aminopeptidase expression and targeting in the insulin-responsive vesicle compartment of glucose transporter isoform 4-deficient cardiomyocytes. Mol. Endocrinol. 18, 2491–2501. 10.1210/me.2004-0175 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

