Brown Adipose Tissue Is Linked to a Distinct Thermoregulatory Response to Mild Cold in People
- PMID: 27148068
- PMCID: PMC4835478
- DOI: 10.3389/fphys.2016.00129
Brown Adipose Tissue Is Linked to a Distinct Thermoregulatory Response to Mild Cold in People
Abstract
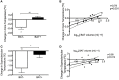
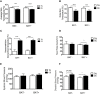
Brown adipose tissue (BAT) plays an important role in thermoregulation in rodents. Its role in temperature homeostasis in people is less studied. To this end, we recruited 18 men [8 subjects with no/minimal BAT activity (BAT-) and 10 with pronounced BAT activity (BAT+)]. Each volunteer participated in a 6 h, individualized, non-shivering cold exposure protocol. BAT was quantified using positron emission tomography/computed tomography. Body core and skin temperatures were measured using a telemetric pill and wireless thermistors, respectively. Core body temperature decreased during cold exposure in the BAT- group only (-0.34°C, 95% CI: -0.6 to -0.1, p = 0.03), while the cold-induced change in core temperature was significantly different between BAT+ and BAT- subjects (BAT+ vs. BAT-, 0.43°C, 95% CI: 0.20-0.65, p = 0.0014). BAT volume was associated with the cold-induced change in core temperature (p = 0.01) even after adjustment for age and adiposity. Compared to the BAT- group, BAT+ subjects tolerated a lower ambient temperature (BAT-: 20.6 ± 0.3°C vs. BAT+: 19.8 ± 0.3°C, p = 0.035) without shivering. The cold-induced change in core temperature (r = 0.79, p = 0.001) and supraclavicular temperature (r = 0.58, p = 0.014) correlated with BAT volume, suggesting that these non-invasive measures can be potentially used as surrogate markers of BAT when other methods to detect BAT are not available or their use is not warranted. These results demonstrate a physiologically significant role for BAT in thermoregulation in people. This trial has been registered with Clinaltrials.gov: NCT01791114 (https://clinicaltrials.gov/ct2/show/NCT01791114).
Keywords: body core temperature; brown adipose tissue; cold exposure; supraclavicular skin temperature; thermoregulation.
Figures


Similar articles
-
Imaging human brown adipose tissue under room temperature conditions with (11)C-MRB, a selective norepinephrine transporter PET ligand.Metabolism. 2015 Jun;64(6):747-55. doi: 10.1016/j.metabol.2015.03.001. Epub 2015 Mar 5. Metabolism. 2015. PMID: 25798999 Free PMC article.
-
Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men.J Physiol. 2015 Feb 1;593(3):701-14. doi: 10.1113/jphysiol.2014.283598. Epub 2014 Dec 15. J Physiol. 2015. PMID: 25384777 Free PMC article.
-
Concurrent validity of supraclavicular skin temperature measured with iButtons and infrared thermography as a surrogate marker of brown adipose tissue.J Therm Biol. 2019 May;82:186-196. doi: 10.1016/j.jtherbio.2019.04.009. Epub 2019 Apr 20. J Therm Biol. 2019. PMID: 31128647
-
Hot heads & cool bodies: The conundrums of human brown adipose tissue (BAT) activity research.Eur J Intern Med. 2017 May;40:26-29. doi: 10.1016/j.ejim.2016.12.023. Epub 2017 Jan 5. Eur J Intern Med. 2017. PMID: 28065662 Review.
-
Does thermoregulatory feeding occur in newborn infants? A novel view of the role of brown adipose tissue thermogenesis in control of food intake.Obes Res. 1995 Jul;3(4):361-9. doi: 10.1002/j.1550-8528.1995.tb00162.x. Obes Res. 1995. PMID: 8521153 Review.
Cited by
-
The life and death of RNA across temperatures.Comput Struct Biotechnol J. 2022 Aug 8;20:4325-4336. doi: 10.1016/j.csbj.2022.08.008. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36051884 Free PMC article. Review.
-
Mitochondrial Energy Metabolism in the Regulation of Thermogenic Brown Fats and Human Metabolic Diseases.Int J Mol Sci. 2023 Jan 10;24(2):1352. doi: 10.3390/ijms24021352. Int J Mol Sci. 2023. PMID: 36674862 Free PMC article. Review.
-
Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease.Front Cardiovasc Med. 2020 Feb 25;7:22. doi: 10.3389/fcvm.2020.00022. eCollection 2020. Front Cardiovasc Med. 2020. PMID: 32158768 Free PMC article. Review.
-
Differences between the most used equations in BAT-human studies to estimate parameters of skin temperature in young lean men.Sci Rep. 2017 Sep 5;7(1):10530. doi: 10.1038/s41598-017-10444-5. Sci Rep. 2017. PMID: 28874709 Free PMC article.
-
Physiological responses to acute cold exposure in young lean men.PLoS One. 2018 May 7;13(5):e0196543. doi: 10.1371/journal.pone.0196543. eCollection 2018. PLoS One. 2018. PMID: 29734360 Free PMC article. Clinical Trial.
References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

