Relationship between Antibiotic Resistance, Biofilm Formation, and Biofilm-Specific Resistance in Acinetobacter baumannii
- PMID: 27148178
- PMCID: PMC4828443
- DOI: 10.3389/fmicb.2016.00483
Relationship between Antibiotic Resistance, Biofilm Formation, and Biofilm-Specific Resistance in Acinetobacter baumannii
Abstract
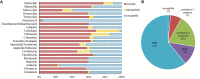
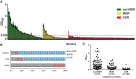
In this study, we aimed to examine the relationships between antibiotic resistance, biofilm formation, and biofilm-specific resistance in clinical isolates of Acinetobacter baumannii. The tested 272 isolates were collected from several hospitals in China during 2010-2013. Biofilm-forming capacities were evaluated using the crystal violet staining method. Antibiotic resistance/susceptibility profiles to 21 antibiotics were assessed using VITEK 2 system, broth microdilution method or the Kirby-Bauer disc diffusion method. The minimum inhibitory concentration (MIC) and minimum biofilm eradication concentration (MBEC) to cefotaxime, imipenem, and ciprofloxacin were evaluated using micro dilution assays. Genetic relatedness of the isolates was also analyzed by pulsed-field gel electrophoresis (PFGE) and plasmid profile. Among all the 272 isolates, 31 were multidrug-resistant (MDR), and 166 were extensively drug-resistant (XDR). PFGE typing revealed 167 pattern types and 103 clusters with a similarity of 80%. MDR and XDR isolates built up the main prevalent genotypes. Most of the non-MDR isolates were distributed in a scattered pattern. Additionally, 249 isolates exhibited biofilm formation, among which 63 were stronger biofilm formers than type strain ATCC19606. Population that exhibited more robust biofilm formation likely contained larger proportion of non-MDR isolates. Isolates with higher level of resistance tended to form weaker biofilms. The MBECs for cefotaxime, imipenem, and ciprofloxacin showed a positive correlation with corresponding MICs, while the enhancement in resistance occurred independent of the quantity of biofilm biomass produced. Results from this study imply that biofilm acts as a mechanism for bacteria to get a better survival, especially in isolates with resistance level not high enough. Moreover, even though biofilms formed by isolates with high level of resistance are always weak, they could still provide similar level of protection for the isolates. Further explorations genetically would improve our understanding of these processes and provide novel insights in the therapeutics and prevention against A. baumannii biofilm-related infections.
Keywords: Acinetobacter baumannii; antibiotic resistance; biofilm; biofilm-specific resistance; pulsed-field gel electrophoresis.
Figures


References
-
- Atashili J., Lyonga E. E., Mandi H., Ikomey G., Mukwele B., Eyoh A. B. (2014). Relationship between multiple drug resistance and biofilm formation in Staphylococcus aureus isolated from medical and non-medical personnel in Yaoundé, Cameroon. Pan Afr. Med. J. 17:186. 10.11604/pamj.2014.17.186.2363 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

