Pyruvate Kinase M2: A Potential Target for Regulating Inflammation
- PMID: 27148264
- PMCID: PMC4838608
- DOI: 10.3389/fimmu.2016.00145
Pyruvate Kinase M2: A Potential Target for Regulating Inflammation
Abstract
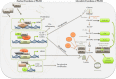
Pyruvate kinase (PK) is the enzyme responsible for catalyzing the last step of glycolysis. Of the four PK isoforms expressed in mammalian cells, PKM2 has generated the most interest due to its impact on changes in cellular metabolism observed in cancer as well as in activated immune cells. As our understanding of dysregulated metabolism in cancer develops, and in light of the growing field of immunometabolism, intense efforts are in place to define the mechanism by which PKM2 regulates the metabolic profile of cancer as well as of immune cells. The enzymatic activity of PKM2 is heavily regulated by endogenous allosteric effectors as well as by intracellular signaling pathways, affecting both the enzymatic activity of PKM2 as a PK and the regulation of the recently described non-canonical nuclear functions of PKM2. We here review the current literature on PKM2 and its regulation, and discuss the potential for this protein as a therapeutic target in inflammatory disorders.
Keywords: HIF-1α; PKM2; cancer; glycolysis and oxidative phosphorylation; immunometabolism; inflammation.
Figures
References
-
- Warburg O. Metabolism of tumours. Biochem Z (1923) 142:317–33.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

