A Complex Molecular Interplay of Auxin and Ethylene Signaling Pathways Is Involved in Arabidopsis Growth Promotion by Burkholderia phytofirmans PsJN
- PMID: 27148317
- PMCID: PMC4828629
- DOI: 10.3389/fpls.2016.00492
A Complex Molecular Interplay of Auxin and Ethylene Signaling Pathways Is Involved in Arabidopsis Growth Promotion by Burkholderia phytofirmans PsJN
Abstract
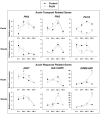
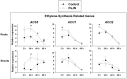
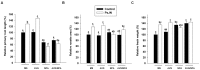
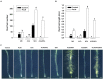
Modulation of phytohormones homeostasis is one of the proposed mechanisms to explain plant growth promotion induced by beneficial rhizobacteria (PGPR). However, there is still limited knowledge about the molecular signals and pathways underlying these beneficial interactions. Even less is known concerning the interplay between phytohormones in plants inoculated with PGPR. Auxin and ethylene are crucial hormones in the control of plant growth and development, and recent studies report an important and complex crosstalk between them in the regulation of different plant developmental processes. The objective of this work was to study the role of both hormones in the growth promotion of Arabidopsis thaliana plants induced by the well-known PGPR Burkholderia phytofirmans PsJN. For this, the spatiotemporal expression patterns of several genes related to auxin biosynthesis, perception and response and ethylene biosynthesis were studied, finding that most of these genes showed specific transcriptional regulations after inoculation in roots and shoots. PsJN-growth promotion was not observed in Arabidopsis mutants with an impaired ethylene (ein2-1) or auxin (axr1-5) signaling. Even, PsJN did not promote growth in an ethylene overproducer (eto2), indicating that a fine regulation of both hormones signaling and homeostasis is necessary to induce growth of the aerial and root tissues. Auxin polar transport is also involved in growth promotion, since PsJN did not promote primary root growth in the pin2 mutant or under chemical inhibition of transport in wild type plants. Finally, a key role for ethylene biosynthesis was found in the PsJN-mediated increase in root hair number. These results not only give new insights of PGPR regulation of plant growth but also are also useful to understand key aspects of Arabidopsis growth control.
Keywords: AVG; EIN2; ETO2; IAA1; NPA; rhizobacteria; root development; root hairs.
Figures

Similar articles
-
Effects of the plant growth-promoting bacterium Burkholderia phytofirmans PsJN throughout the life cycle of Arabidopsis thaliana.PLoS One. 2013 Jul 15;8(7):e69435. doi: 10.1371/journal.pone.0069435. Print 2013. PLoS One. 2013. PMID: 23869243 Free PMC article.
-
Quorum sensing and indole-3-acetic acid degradation play a role in colonization and plant growth promotion of Arabidopsis thaliana by Burkholderia phytofirmans PsJN.Mol Plant Microbe Interact. 2013 May;26(5):546-53. doi: 10.1094/MPMI-10-12-0241-R. Mol Plant Microbe Interact. 2013. PMID: 23301615
-
Burkholderia phytofirmans PsJN induces long-term metabolic and transcriptional changes involved in Arabidopsis thaliana salt tolerance.Front Plant Sci. 2015 Jun 23;6:466. doi: 10.3389/fpls.2015.00466. eCollection 2015. Front Plant Sci. 2015. PMID: 26157451 Free PMC article.
-
Deciphering Auxin-Ethylene Crosstalk at a Systems Level.Int J Mol Sci. 2018 Dec 14;19(12):4060. doi: 10.3390/ijms19124060. Int J Mol Sci. 2018. PMID: 30558241 Free PMC article. Review.
-
Paraburkholderia phytofirmans PsJN-Plants Interaction: From Perception to the Induced Mechanisms.Front Microbiol. 2018 Aug 30;9:2093. doi: 10.3389/fmicb.2018.02093. eCollection 2018. Front Microbiol. 2018. PMID: 30214441 Free PMC article. Review.
Cited by
-
Comparative transcriptome profiling provides insights into the growth promotion activity of Pseudomonas fluorescens strain SLU99 in tomato and potato plants.Front Plant Sci. 2023 Jul 18;14:1141692. doi: 10.3389/fpls.2023.1141692. eCollection 2023. Front Plant Sci. 2023. PMID: 37534284 Free PMC article.
-
Comparative Transcriptomics and Metabolomics Reveal an Intricate Priming Mechanism Involved in PGPR-Mediated Salt Tolerance in Tomato.Front Plant Sci. 2021 Aug 17;12:713984. doi: 10.3389/fpls.2021.713984. eCollection 2021. Front Plant Sci. 2021. PMID: 34484277 Free PMC article.
-
What do we know from the transcriptomic studies investigating the interactions between plants and plant growth-promoting bacteria?Front Plant Sci. 2022 Sep 15;13:997308. doi: 10.3389/fpls.2022.997308. eCollection 2022. Front Plant Sci. 2022. PMID: 36186072 Free PMC article. Review.
-
Transcriptome profiling of genes involved in induced systemic salt tolerance conferred by Bacillus amyloliquefaciens FZB42 in Arabidopsis thaliana.Sci Rep. 2017 Sep 13;7(1):10795. doi: 10.1038/s41598-017-11308-8. Sci Rep. 2017. PMID: 28904348 Free PMC article.
-
Paraburkholderia phytofirmans PsJN triggers local and systemic transcriptional reprogramming in Arabidopsis thaliana and increases resistance against Botrytis cinerea.Front Plant Sci. 2025 Jun 3;16:1554036. doi: 10.3389/fpls.2025.1554036. eCollection 2025. Front Plant Sci. 2025. PMID: 40530279 Free PMC article.
References
-
- Ahmed A., Hasnain S. (2014). Auxins as one of the factors of plant growth improvement by plant growth promoting rhizobacteria. Pol. J. Microbiol. 63 261–266. - PubMed
-
- Babula D., Misztal L. H., Jakubowicz M., Kaczmarek M., Nowak W., Sadowski J. (2006). Genes involved in biosynthesis and signalisation of ethylene in Brassica oleracea and Arabidopsis thaliana: identification and genome comparative mapping of specific gene homologues. Theor. Appl. Genet. 112 410–420. 10.1007/s00122-005-0136-7 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

