Immune Responses to Single-Dose Versus Double-Dose Hepatitis B Vaccines in Healthcare Workers not Responding to the Primary Vaccine Series: A Randomized Clinical Trial
- PMID: 27148385
- PMCID: PMC4852093
- DOI: 10.5812/hepatmon.32799
Immune Responses to Single-Dose Versus Double-Dose Hepatitis B Vaccines in Healthcare Workers not Responding to the Primary Vaccine Series: A Randomized Clinical Trial
Abstract
Background: Recommendations to immunize healthcare workers (HCWs) against hepatitis B are well known. However, a proportion of individuals do not respond to the primary standard three-dose HB vaccination schedule.
Objectives: The current study aimed to evaluate whether a double-dose HB booster vaccine could induce better protective anti-HB titers than a single-dose booster in non-protected HCWs.
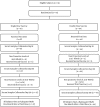
Materials and methods: This was a randomized clinical trial. A total of 91 HCWs not responding to the primary vaccine series in 2014 were enrolled. The participants were randomized into two groups that received a double dose of the HB vaccine containing 40 µg of antigen or a single dose of the HB vaccine containing 20 µg of antigen in three doses (at zero, one and six months after vaccination). Blood samples were collected before vaccinations and 28 days after the third dose to assess the seroconversion rate, according to the anti-HB antibody titer threshold of > 10 mIU/mL.
Results: The seroconversion rates were 93.2% and 87.2% after the first booster doses of the double-dose and single-dose HB vaccines, respectively (P = 0.64). In the double-dose HB vaccine group, the seroconversion rate was 97.8% compared with 89.6% in the single-dose group following the second vaccine dose (P = 0.83). All of the participants in both groups were seroprotected after the third HB vaccine dose.
Conclusions: Both the single- and double-dose HB vaccines were adequately immunogenic, and the double-dose HB vaccine was not significantly more immunogenic than the single-dose vaccine in terms of the seroconversion rates of HCWs who had not responded to the primary vaccine series.
Keywords: Healthcare; Healthcare Personnel; Hepatitis B Vaccine; Immune Response Antigens; Immunogenicity.
Figures
References
-
- Ghany MG, Perrillo R, Li R, Belle SH, Janssen HL, Terrault NA, et al. Characteristics of adults in the hepatitis B research network in North America reflect their country of origin and hepatitis B virus genotype. Clin Gastroenterol Hepatol. 2015;13(1):183–92. doi: 10.1016/j.cgh.2014.06.028. - DOI - PMC - PubMed
-
- Lavanchy D. Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention. J Clin Virol. 2005;34 Suppl 1:S1–3. - PubMed
-
- Centers for Disease Control and Prevention. CDC; Available from: http://www.cdc.gov/.
LinkOut - more resources
Full Text Sources
Other Literature Sources