Oxygen Supplementation to Stabilize Preterm Infants in the Fetal to Neonatal Transition: No Satisfactory Answer
- PMID: 27148504
- PMCID: PMC4835680
- DOI: 10.3389/fped.2016.00029
Oxygen Supplementation to Stabilize Preterm Infants in the Fetal to Neonatal Transition: No Satisfactory Answer
Abstract
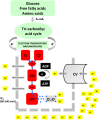
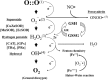
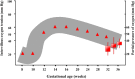
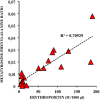
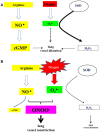
Fetal life elapses in a relatively low oxygen environment. Immediately after birth with the initiation of breathing, the lung expands and oxygen availability to tissue rises by twofold, generating a physiologic oxidative stress. However, both lung anatomy and function and the antioxidant defense system do not mature until late in gestation, and therefore, very preterm infants often need respiratory support and oxygen supplementation in the delivery room to achieve postnatal stabilization. Notably, interventions in the first minutes of life can have long-lasting consequences. Recent trials have aimed to assess what initial inspiratory fraction of oxygen and what oxygen targets during this transitional period are best for extremely preterm infants based on the available nomogram. However, oxygen saturation nomogram informs only of term and late preterm infants but not on extremely preterm infants. Therefore, the solution to this conundrum may still have to wait before a satisfactory answer is available.
Keywords: biomarkers; free radicals; oxidative stress; oxygen; oxygen saturation; prematurity; pulse oximetry.
Figures
Similar articles
-
Oxygen Supplementation During Preterm Stabilization and the Relevance of the First 5 min After Birth.Front Pediatr. 2020 Jan 31;8:12. doi: 10.3389/fped.2020.00012. eCollection 2020. Front Pediatr. 2020. PMID: 32083039 Free PMC article. Review.
-
The Respiratory Management of the Extreme Preterm in the Delivery Room.Children (Basel). 2023 Feb 10;10(2):351. doi: 10.3390/children10020351. Children (Basel). 2023. PMID: 36832480 Free PMC article. Review.
-
Oxygen supplementation in the neonatal period: changing the paradigm.Neonatology. 2014;105(4):323-31. doi: 10.1159/000360646. Epub 2014 May 30. Neonatology. 2014. PMID: 24931324 Review.
-
Targeting Oxygen in Term and Preterm Infants Starting at Birth.Clin Perinatol. 2019 Sep;46(3):459-473. doi: 10.1016/j.clp.2019.05.013. Epub 2019 Jun 12. Clin Perinatol. 2019. PMID: 31345541 Review.
-
Oxygen administration to preterm neonates in the delivery room: minimizing oxidative stress.Adv Neonatal Care. 2015 Apr;15(2):94-103; quiz E1-2. doi: 10.1097/ANC.0000000000000147. Adv Neonatal Care. 2015. PMID: 25822515 Review.
Cited by
-
Oxygen and oxidative stress in the perinatal period.Redox Biol. 2017 Aug;12:674-681. doi: 10.1016/j.redox.2017.03.011. Epub 2017 Mar 12. Redox Biol. 2017. PMID: 28395175 Free PMC article. Review.
-
Intermittent hypoxemia and oxidative stress in preterm infants.Respir Physiol Neurobiol. 2019 Aug;266:121-129. doi: 10.1016/j.resp.2019.05.006. Epub 2019 May 14. Respir Physiol Neurobiol. 2019. PMID: 31100375 Free PMC article. Review.
-
Impact of the Neonatal Resuscitation Program-Recommended Low Oxygen Strategy on Outcomes of Infants Born Preterm.J Pediatr. 2017 Dec;191:35-41. doi: 10.1016/j.jpeds.2017.08.074. J Pediatr. 2017. PMID: 29173319 Free PMC article.
-
Inhaled Nitric Oxide at Birth Reduces Pulmonary Vascular Resistance and Improves Oxygenation in Preterm Lambs.Children (Basel). 2021 May 11;8(5):378. doi: 10.3390/children8050378. Children (Basel). 2021. PMID: 34064629 Free PMC article.
-
Optimal Inspired Fraction of Oxygen in the Delivery Room for Preterm Infants.Children (Basel). 2019 Feb 19;6(2):29. doi: 10.3390/children6020029. Children (Basel). 2019. PMID: 30791491 Free PMC article. Review.
References
-
- Vento M, Hummler H, Dawson JA, Escobar JJ, Kuligowski J. Use of oxygen in the resuscitation of neonates. 1st ed In: Dennery PA, et al., editor. Perinatal and Prenatal Disorders. Oxidative Stress in Applied Basic Research and Clinical Practice. New York: Springer Science + Business Media; (2015). p. 213–43.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

