Clusterin facilitates apoptotic cell clearance and prevents apoptotic cell-induced autoimmune responses
- PMID: 27148688
- PMCID: PMC4917652
- DOI: 10.1038/cddis.2016.113
Clusterin facilitates apoptotic cell clearance and prevents apoptotic cell-induced autoimmune responses
Abstract
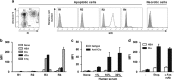
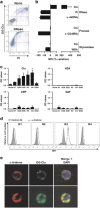
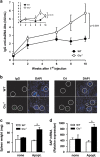
Clusterin (Clu), an extracellular chaperone, exhibits characteristics of soluble innate immunity receptors, as assessed by its ability to bind some bacteria strains. In this study, we report that Clu also binds specifically to late apoptotic cells but not to live, early apoptotic, or necrotic cells. Histones, which accumulate on blebs during the apoptotic process, represent privileged Clu-binding motifs at the surface of late apoptotic cells. As a consequence, Clu potentiates, both in vitro and in vivo, the phagocytosis of late apoptotic cells by macrophages. Moreover, the increased phagocytosis of late apoptotic cells induced by Clu favors the presentation and cross-presentation of apoptotic cell-associated antigens. Finally, we observed that, in a model of apoptotic cell-induced autoimmunity, and relative to control mice, Clu(-/-) mice develop symptoms of autoimmunity, including the generation of anti-dsDNA antibodies, deposition of immunoglobulins and complement components within kidneys, and splenomegaly. These results identify Clu as a new molecule partner involved in apoptotic cell efferocytosis and suggest a protective role for Clu in inflammation and autoimmune diseases.
Figures




References
-
- Trougakos IP, Gonos ES. Clusterin/apolipoprotein J in human aging and cancer. Int J Biochem Cell Biol 2002; 34: 1430–1448. - PubMed
-
- Jenne DE, Lowin B, Peitsch MC, Bottcher A, Schmitz G, Tschopp J. Clusterin (complement lysis inhibitor) forms a high density lipoprotein complex with apolipoprotein A-I in human plasma. J Biol Chem 1991; 266: 11030–11036. - PubMed
-
- Hogasen K, Mollnes TE, Tschopp J, Harboe M. Quantitation of vitronectin and clusterin. Pitfalls and solutions in enzyme immunoassays for adhesive proteins. J Immunol Methods 1993; 160: 107–115. - PubMed
-
- Morrissey C, Lakins J, Moquin A, Hussain M, Tenniswood M. An antigen capture assay for the measurement of serum clusterin concentrations. J Biochem Biophys Methods 2001; 48: 13–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

